
Am Fam Physician. 2015;92(8):732-740
Key Points for Practice
• Children six months to eight years of age who have received at least two doses of trivalent or quadrivalent influenza vaccine since the 2010–2011 influenza season need only one dose this season.
• Live attenuated influenza vaccine is no longer recommended over inactivated vaccine for children two to eight years of age.
• Persons with a history of egg allergy who have experienced only hives after exposure to egg should receive inactivated influenza vaccine or trivalent recombinant influenza vaccine.
From the AFP Editors
The Centers for Disease Control and Prevention's Advisory Committee on Immunization Practices (ACIP) has released its yearly recommendations for routine influenza vaccination in the 2015–2016 season. Updates this year include the antigenic composition of seasonal influenza vaccines available in the United States; information on influenza vaccines expected to be available this season; updated information for determining the number of doses required for children six months to eight years of age; and recommendations for the use of live attenuated influenza vaccine (LAIV) when both LAIV and inactivated influenza vaccine are available, including the removal of 2014–2015 preferential recommendation for LAIV in healthy children two to eight years of age.
Routine annual influenza vaccination is recommended for all persons six months and older who do not have contraindications. Vaccination should ideally occur before the onset of influenza activity in the community. Clinicians should offer vaccination by October, if possible, and continue through the influenza season. Children six months to eight years of age who require two doses should receive their first dose as soon as possible after vaccine becomes available, and the second dose no earlier than four weeks later.
For the 2015–2016 influenza season, U.S.-licensed trivalent influenza vaccines will include hemagglutinin derived from an A/California/7/2009 (H1N1)-like virus, an A/Switzerland/9715293/2013 (H3N2)-like virus, and a B/Phuket/3073/2013-like (Yamagata lineage) virus. Quadrivalent vaccines will contain these viruses plus a B/ Brisbane/60/2008-like (Victoria lineage) virus.
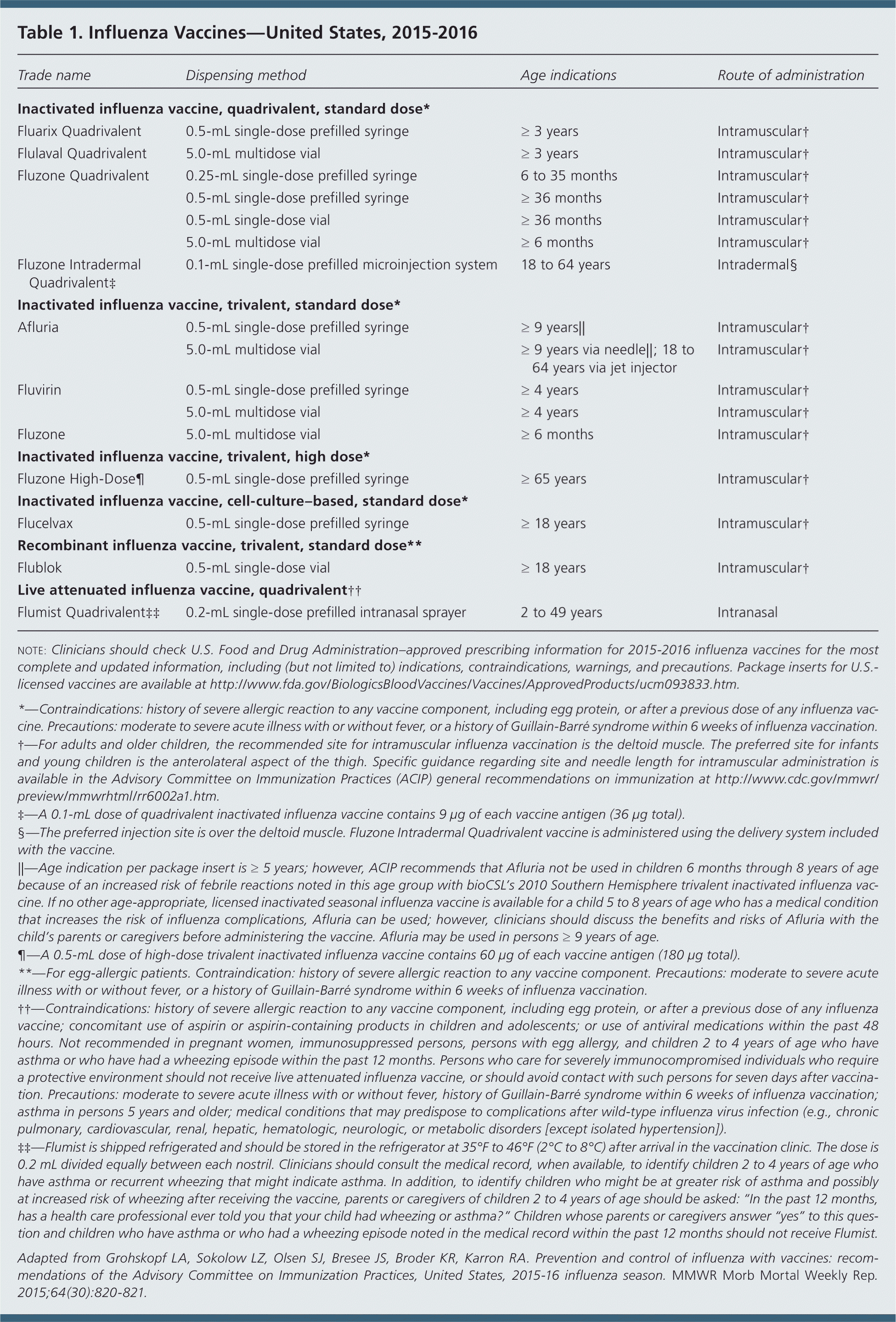
Influenza vaccines expected to be available this season are listed in Table 1. New vaccines and updated vaccine indications include the following:
The trivalent inactivated influenza vaccine Afluria has been approved for intramuscular administration via a needle-free jet injector in persons 18 to 64 years of age. Afluria is the only inactivated influenza vaccine that can be administered without a needle and syringe.
The trivalent recombinant influenza vaccine, Flublok, (for persons with egg allergy) is now indicated for all adults 18 years and older. It was previously approved only for persons 18 to 49 years of age.
The quadrivalent intradermal inactivated influenza vaccine, Fluzone Intradermal, is now indicated for adults 18 to 64 years of age. This formulation is expected to replace the previously available trivalent intradermal inactivated vaccine.
| Trade name | Dispensing method | Age indications | Route of administration |
|---|---|---|---|
| Inactivated influenza vaccine, quadrivalent, standard dose* | |||
| Fluarix Quadrivalent | 0.5-mL single-dose prefilled syringe | ≥ 3 years | Intramuscular† |
| Flulaval Quadrivalent | 5.0-mL multidose vial | ≥ 3 years | Intramuscular† |
| Fluzone Quadrivalent | 0.25-mL single-dose prefilled syringe | 6 to 35 months | Intramuscular† |
| 0.5-mL single-dose prefilled syringe | ≥ 36 months | Intramuscular† | |
| 0.5-mL single-dose vial | ≥ 36 months | Intramuscular† | |
| 5.0-mL multidose vial | ≥ 6 months | Intramuscular† | |
| Fluzone Intradermal Quadrivalent‡ | 0.1-mL single-dose prefilled microinjection system | 18 to 64 years | Intradermal§ |
| Inactivated influenza vaccine, trivalent, standard dose* | |||
| Afluria | 0.5-mL single-dose prefilled syringe | ≥ 9 years|| | Intramuscular† |
| 5.0-mL multidose vial | ≥ 9 years via needle||; 18 to 64 years via jet injector | Intramuscular† | |
| Fluvirin | 0.5-mL single-dose prefilled syringe | ≥ 4 years | Intramuscular† |
| 5.0-mL multidose vial | ≥ 4 years | Intramuscular† | |
| Fluzone | 5.0-mL multidose vial | ≥ 6 months | Intramuscular† |
| Inactivated influenza vaccine, trivalent, high dose* | |||
| Fluzone High-Dose¶ | 0.5-mL single-dose prefilled syringe | ≥ 65 years | Intramuscular† |
| Inactivated influenza vaccine, cell-culture–based, standard dose* | |||
| Flucelvax | 0.5-mL single-dose prefilled syringe | ≥ 18 years | Intramuscular† |
| Recombinant influenza vaccine, trivalent, standard dose** | |||
| Flublok | 0.5-mL single-dose vial | ≥ 18 years | Intramuscular† |
| Live attenuated influenza vaccine, quadrivalent†† | |||
| Flumist Quadrivalent‡‡ | 0.2-mL single-dose prefilled intranasal sprayer | 2 to 49 years | Intranasal |
Children six months to eight years of age require two doses of influenza vaccine during their first season of vaccination. Since the emergence of influenza A(H1N1)pdm09 (the 2009 H1N1 pandemic virus), recommendations for determining the number of doses needed have been based on whether a child previously received vaccine containing influenza A(H1N1)pdm09. Because this strain continues to circulate as the predominant H1N1 virus, and because of the inclusion of an A/California/7/2009(H1N1)-like virus in seasonal influenza vaccines available in the United States since the 2010–2011 season, separate consideration of receipt of vaccine doses containing this virus is no longer recommended. Children six months to eight years of age who received at least two doses of trivalent or quadrivalent influenza vaccine before July 1, 2015, need only one dose this season. The two previous doses do not have to have been given during the same season or consecutive seasons.
LAIV and inactivated influenza vaccine have both been proven effective in children and adults. Although ACIP previously recommended that LAIV be given to healthy children two to eight years of age, recent evidence has shown that LAIV is no more effective than inactivated influenza vaccine. Therefore, LAIV is no longer recommended over inactivated vaccine; when both vaccines are available in an age-appropriate formulation, either can be given.
Egg Allergy
Severe allergic and anaphylactic reactions can occur in response to various components of influenza vaccine, but such reactions are rare. All currently available influenza vaccines except trivalent recombinant influenza vaccine and cell-culture–based inactivated influenza vaccine (Flucelvax) are prepared by propagation of virus in embryonated eggs. For the 2015–2016 influenza season, ACIP recommends the following:
Persons with a history of egg allergy who have experienced only hives after exposure to egg should receive inactivated influenza vaccine or trivalent recombinant influenza vaccine. Recombinant vaccine can be used in adults 18 years and older who have no contraindications. However, inactivated vaccine may also be used if it is administered by a clinician who is familiar with the potential manifestations of egg allergy and if the patient can be observed for signs of a reaction for at least 30 minutes after vaccination.
Persons with a history of symptoms such as angioedema, respiratory distress, light-headedness, or recurrent emesis after exposure to egg, or who required epinephrine or another emergency medical intervention, may receive recombinant influenza vaccine if they are at least 18 years of age and have no other contraindications. If recombinant vaccine is not available or the recipient is not within the indicated age range, inactivated influenza vaccine should be administered by a clinician experienced in the recognition and management of severe allergic reactions.
Regardless of allergy history, all vaccines should be administered in settings in which personnel and equipment for rapid recognition and treatment of anaphylaxis are available.
Persons who can eat lightly cooked egg (e.g., scrambled egg) without reaction are unlikely to be allergic. Egg-allergic persons may tolerate egg in baked products (e.g., bread, cake). Tolerance to egg-containing foods does not exclude the possibility of egg allergy. Egg allergy can be confirmed by a consistent medical history of adverse reactions to eggs and egg-containing foods, plus skin or blood testing for immunoglobulin E directed against egg proteins.
In persons with no history of egg exposure who are suspected of being allergic on the basis of allergy testing, consultation with a clinician who has expertise in the management of allergic conditions should be obtained before vaccination. Alternatively, trivalent recombinant influenza vaccine can be administered if the patient is at least 18 years of age.
A history of severe allergic reaction to influenza vaccine is a contraindication to future receipt of the vaccine, regardless of the component suspected of causing the reaction.
Guideline source: Advisory Committee on Immunization Practices
Evidence rating system used? No
Literature search described? No
Guideline developed by participants without relevant financial ties to industry? Not reported
Published source: MMWR Morb Mortal Wkly Rep, August, 7, 2015;64(30):818–825
Available at: http://www.cdc.gov/mmwr/preview/mmwrhtml/mm6430a3.htm
