
This is a corrected version of the article that appeared in print.
Am Fam Physician. 2016;93(2):103-109
Related U.S. Preventive Services Task Force recommendation statement: Screening for Abnormal Blood Glucose and Type 2 Diabetes Mellitus: Recommendation Statement
Author disclosure: No relevant financial affiliations.
Diabetes mellitus is one of the most common diagnoses made by family physicians. Uncontrolled diabetes can lead to blindness, limb amputation, kidney failure, and vascular and heart disease. Screening patients before signs and symptoms develop leads to earlier diagnosis and treatment, but may not reduce rates of end-organ damage. Randomized trials show that screening for type 2 diabetes does not reduce mortality after 10 years, although some data suggest mortality benefits after 23 to 30 years. Lifestyle and pharmacologic interventions decrease progression to diabetes in patients with impaired fasting glucose or impaired glucose tolerance. Screening for type 1 diabetes is not recommended. The U.S. Preventive Services Task Force recommends screening for abnormal blood glucose and type 2 diabetes in adults 40 to 70 years of age who are overweight or obese, and repeating testing every three years if results are normal. Individuals at higher risk should be considered for earlier and more frequent screening. The American Diabetes Association recommends screening for type 2 diabetes annually in patients 45 years and older, or in patients younger than 45 years with major risk factors. The diagnosis can be made with a fasting plasma glucose level of 126 mg per dL or greater; an A1C level of 6.5% or greater; a random plasma glucose level of 200 mg per dL or greater; or a 75-g two-hour oral glucose tolerance test with a plasma glucose level of 200 mg per dL or greater. Results should be confirmed with repeat testing on a subsequent day; however, a single random plasma glucose level of 200 mg per dL or greater with typical signs and symptoms of hyperglycemia likely indicates diabetes. Additional testing to determine the etiology of diabetes is not routinely recommended.
Diabetes mellitus is a group of metabolic diseases characterized by hyperglycemia resulting from defects in insulin secretion, insulin action, or both.1,2 Uncontrolled diabetes can lead to blindness, limb amputation, kidney failure, vascular disease, and heart disease. It is estimated that in the next 20 years, the number of persons with type 2 diabetes in the United States will reach 44 million, approximately double the current prevalence.3 Diabetes likely will continue to be one of the most common diagnoses made by family physicians.4 Diagnostic testing should be performed in individuals with a clinical history indicative of diabetes. Symptoms that should prompt consideration of diabetes include polyuria, polydipsia, fatigue, blurry vision, weight loss, poor wound healing, numbness, and tingling. This article focuses on screening and diagnosis of diabetes in asymptomatic patients.
| Clinical recommendation | Evidence rating | References |
|---|---|---|
| Treatment of impaired fasting glucose and impaired glucose tolerance with pharmacologic interventions, lifestyle interventions, or both decreases progression to diabetes mellitus. | C | 7, 8 |
| Patients 40 to 70 years of age who are overweight or obese should be screened for type 2 diabetes. Persons with abnormal results should be referred for intensive behavioral counseling interventions that focus on physical activity and a healthy diet. | B | 7, 8 |
| If initial screening results for type 2 diabetes are normal, screening may be repeated every three years. | C | 7, 8 |
| Diagnosis of type 2 diabetes can be made using fasting plasma glucose, A1C testing, random plasma glucose testing, or an oral glucose tolerance test. | C | 1, 2 |
Classifying Diabetes
Type 1 diabetes is caused by autoimmune destruction of the islet cells of the pancreas, and onset is typically in childhood. Type 2 diabetes is caused by insulin resistance and is more common in patients who are obese.2 Previously thought to primarily affect adults, type 2 diabetes is now being diagnosed more often in children and adolescents with obesity. End-organ damage and complications are similar in both types of diabetes.
TYPE 1 DIABETES
Screening for type 1 diabetes is not recommended for the following reasons: patients typically present with an acute onset of symptoms, no established cutoff value is available for antibody tests, no accepted treatment exists for patients who are asymptomatic, and no medication is available to prevent the disease in persons genetically predisposed to type 1 diabetes.5,6
TYPE 2 DIABETES
Although screening for type 2 diabetes does not improve mortality after 10 years of follow-up,9,10 studies show that lifestyle and pharmacologic interventions in patients with impaired glucose tolerance and impaired fasting glucose can delay development of type 2 diabetes,11 with some studies showing greater effectiveness with lifestyle changes.12,13 Other studies suggest screening may begin to show benefits in mortality after 23 to 30 years.14,15 One randomized trial showed a statistically significant reduction in the incidence of all-cause and cardiovascular mortality in patients with impaired glucose tolerance treated with lifestyle modifications, although only after 23 years of follow-up (not found at 20-year evaluation). This study was conducted in China and may not be applicable to a U.S. population.15
Who Should Be Screened
NONPREGNANT ADULTS
Multiple professional organizations have published screening recommendations for type 2 diabetes, although slight differences exist (Table 1).8,16–20 The U.S. Preventive Services Task Force (USPSTF) recently updated recommendations and suggests screening individuals 40 to 70 years of age who are overweight or obese. Persons with abnormal results should be referred for intensive behavioral counseling interventions focusing on physical activity and a healthy diet. Clinicians should consider screening certain individuals at higher risk.7,8 The USPSTF relied on evidence from randomized trials to identify populations who would be most likely to benefit from screening. Based on cohort studies, the American Diabetes Association (ADA) recommends screening a broader population based on risk, including all adults 45 years or older regardless of risk, and includes screening for prediabetes in the guidelines.6,21 There are multiple risk prediction calculators available,22–24 although most prediction models overestimate diabetes risk.9 However, the Canadian Task Force on Preventive Health Care recommends using one of two validated risk questionnaires to help determine who should be screened.18

| American Association of Clinical Endocrinologists16 | |
| Screen asymptomatic individuals if risk factors present: | |
| Acanthosis nigricans | |
| Age ≥ 45 years | |
| Antipsychotic therapy for schizophrenia and/or severe bipolar disease | |
| Cardiovascular disease or family history of type 2 diabetes | |
| Chronic glucocorticoid exposure | |
| HDL cholesterol level < 35 mg per dL (0.91 mmol per L) and/or a triglyceride level > 250 mg per dL (2.8 mmol per L) | |
| History of gestational diabetes mellitus or delivery of a baby weighing > 9 lb (4.1 kg) | |
| Hypertension (blood pressure > 140/90 mm Hg or taking medication for hypertension) | |
| Impaired glucose tolerance, impaired fasting glucose, and/or metabolic syndrome | |
| Member of an at-risk racial or ethnic group: Asian, black, Hispanic, Native American (Alaska Native or American Indian), or Pacific Islander | |
| Nonalcoholic fatty liver disease | |
| Overweight or obese | |
| Polycystic ovary syndrome | |
| Sedentary lifestyle | |
| Sleep disorders in the presence of glucose intolerance (A1C > 5.7%, impaired glucose tolerance, or impaired fasting glucose on previous testing), including obstructive sleep apnea, chronic sleep deprivation, and night-shift occupation) every three years | |
| Screen persons with two or more risk factors annually | |
| American Diabetes Association17 | |
| Screen asymptomatic adults with a body mass index ≥ 25 kg per m2, and one or more additional risk factors: | |
| A1C > 5.7%, impaired glucose tolerance, or impaired fasting glucose on previous testing | |
| Acanthosis nigricans | |
| Cardiovascular disease | |
| First-degree relative with type 2 diabetes | |
| HDL cholesterol level < 35 mg per dL and/or a triglyceride level > 250 mg per dL | |
| High-risk ethnicity: black, Native American/Alaska Native, Hispanic/Latino, Asian American, and Native Hawaiian/Pacific Islander | |
| Hypertension (blood pressure > 140/90 mm Hg or taking medication for hypertension) | |
| Physical inactivity | |
| Polycystic ovary syndrome | |
| Women who had gestational diabetes or who delivered a baby weighing > 9 lb | |
| In persons without risk factors, testing should begin at 45 years of age | |
| If test results are normal, repeat testing should be performed at least every three years | |
| Canadian Task Force on Preventive Health Care18 | |
| Screening is not recommended for adults at low to moderate risk of diabetes (risk determined with a validated risk calculator: FINDRISC19 and CANRISK,20 which factor in age, obesity, history of elevated glucose levels, history of hypertension, family history of diabetes, limited activity levels, and diet with limited intake of fruits and vegetables) | |
| For adults at high risk of diabetes, routine screening every three to five years with A1C | |
| For adults at very high risk of diabetes, routine screening annually with A1C | |
| U.S. Preventive Services Task Force8 | |
| Screen all adults 40 to 70 years of age who are overweight or obese (grade B) | |
| Consider screening earlier in patients with higher risk (i.e., one of the following): family history of diabetes; members of certain racial and ethnic groups (i.e., blacks, American Indians or Alaska Natives, Asian Americans, Hispanics or Latinos, or Native Hawaiians or Pacific Islanders); personal history of gestational diabetes or polycystic ovary syndrome | |
PREGNANT WOMEN
Hyperglycemia increases the risk of congenital malformations and intrauterine fetal death. Women with gestational diabetes mellitus (GDM) who have fasting hyperglycemia have a three- to fourfold increased risk of infant malformations.26,27 The goal of screening is to reduce maternal and fetal complications such as preeclampsia, cesarean delivery, congenital malformations, macrosomia (and later childhood/adolescent overweight), shoulder dystocia, nerve palsy, bone fracture, jaundice, and infant death.26,28–30
The ADA advises screening pregnant women in their first trimester if they have risk factors for developing type 2 diabetes (Table 18,16–20 ) or GDM, including obesity, advanced maternal age (older than 35 years), history of GDM, family history of diabetes, and belonging to a high-risk ethnic group.17 The American College of Obstetricians and Gynecologists and the Centers for Disease Control and Prevention agree with this recommendation.31,32 However, the American Academy of Family Physicians and the USPSTF recommend screening for GDM only after 24 weeks' gestation.29,33,34
Screening for GDM should be performed using a two-step 50-g nonfasting oral glucose challenge test; if the result is positive, this is followed by a diagnostic 100-g fasting oral glucose tolerance test.34 Further information about screening and diagnosis of GDM is available in a previous article in American Family Physician (https://www.aafp.org/afp/2015/0401/p460.html).
CHILDREN
The ADA recommends screening children and adolescents 18 years and younger who are overweight (i.e., body mass index greater than 85th percentile for age and sex, weight for height greater than 85th percentile, or weight greater than 120% of ideal [50th percentile] for height) and who have any two of the following risk factors: history of type 2 diabetes in a first- or second-degree relative, belonging to a high-risk ethnic group (Table 18,16–20 ), acanthosis nigricans, hypertension, hyperlipidemia, or polycystic ovary syndrome.35 The American Academy of Pediatrics and the ADA recommend screening at-risk patients every two years starting at 10 years of age, or at onset of puberty if before 10 years of age.36,37
OLDER ADULTS
Although more than 50% of older adults have prediabetes, and older adults in general are at higher risk of prediabetes and type 2 diabetes, the benefits of screening depend on whether treatment would improve the patient's overall quality of life or life expectancy.38 No organizations currently recommend routine screening in geriatric patients, although the ADA does support the consideration of screening to prevent complications that could lead to functional impairment. Although treatment goals may differ in older patients, diagnostic thresholds are the same.39 The age at which to discontinue screening has not been established, but should be a shared decision between the physician and patient based on life expectancy and other comorbidities.38
Diagnostic Testing
The diagnosis of diabetes can be made when classic signs and symptoms of hyperglycemia are associated with a single random plasma glucose measurement of 200 mg per dL (11.1 mmol per L) or greater. Alternatively, the diagnosis can be made with an A1C level of 6.5% or greater, a fasting plasma glucose level of 126 mg per dL (7.0 mmol per L) or greater, or a two-hour plasma glucose level of 200 mg per dL or greater during an oral glucose tolerance test with 75-g glucose load (Table 217 ); however, testing should be repeated on a subsequent day to confirm the diagnosis.1,17 If testing results do not match the clinical picture or are inconsistent, repeat testing or testing with another modality may be helpful.17 [corrected]
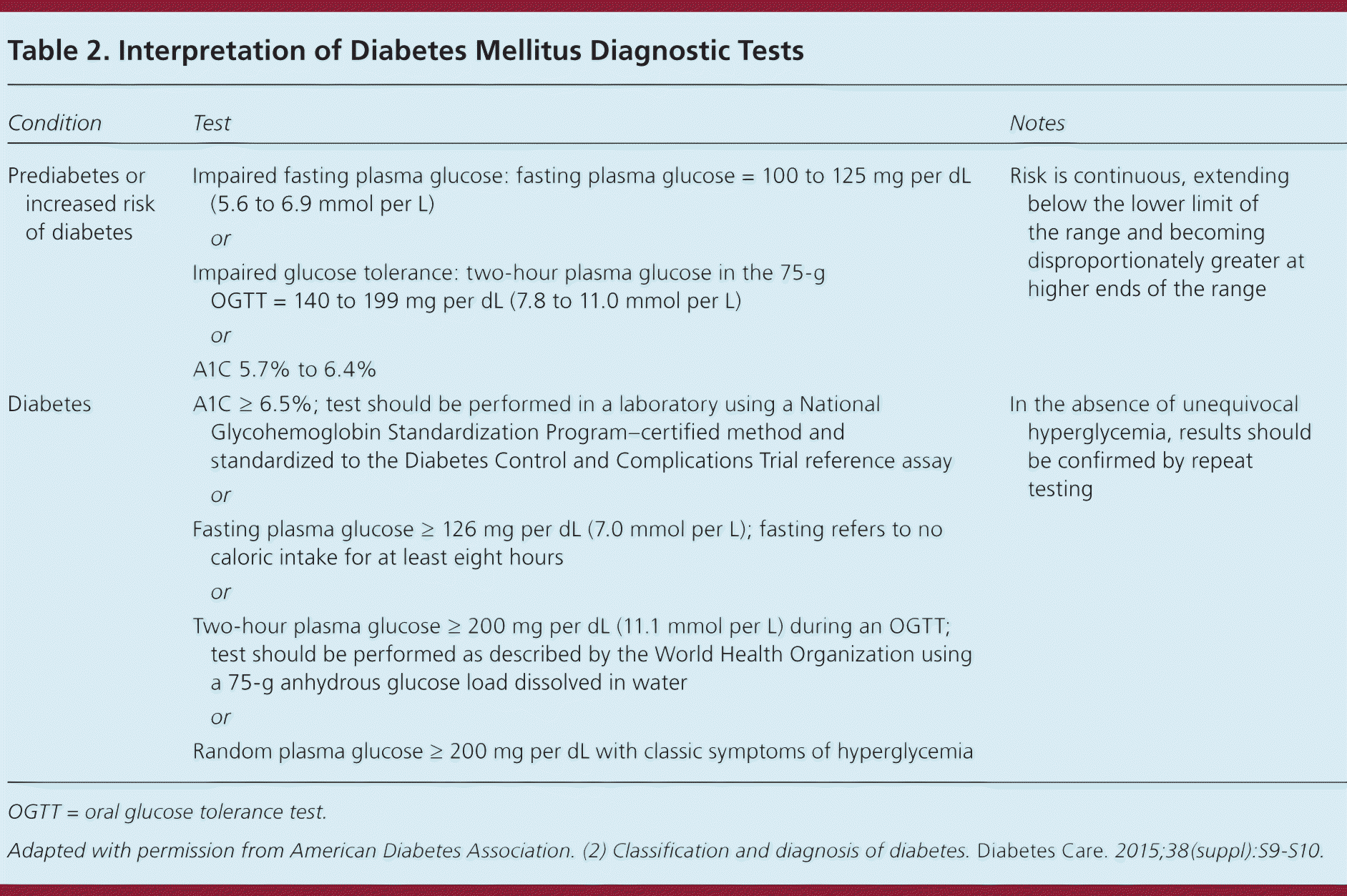
| Condition | Test | Notes | |
|---|---|---|---|
| Prediabetes or increased risk of diabetes | Impaired fasting plasma glucose: fasting plasma glucose = 100 to 125 mg per dL (5.6 to 6.9 mmol per L) | Risk is continuous, extending below the lower limit of the range and becoming disproportionately greater at higher ends of the range | |
| or | |||
| Impaired glucose tolerance: two-hour plasma glucose in the 75-g OGTT = 140 to 199 mg per dL (7.8 to 11.0 mmol per L) | |||
| or | |||
| A1C 5.7% to 6.4% | |||
| Diabetes | A1C ≥ 6.5%; test should be performed in a laboratory using a National Glycohemoglobin Standardization Program–certified method and standardized to the Diabetes Control and Complications Trial reference assay | In the absence of unequivocal hyperglycemia, results should be confirmed by repeat testing | |
| or | |||
| Fasting plasma glucose ≥ 126 mg per dL (7.0 mmol per L); fasting refers to no caloric intake for at least eight hours | |||
| or | |||
| Two-hour plasma glucose ≥ 200 mg per dL (11.1 mmol per L) during an OGTT; test should be performed as described by the World Health Organization using a 75-g anhydrous glucose load dissolved in water | |||
| or | |||
| Random plasma glucose ≥ 200 mg per dL with classic symptoms of hyperglycemia | |||
A1C LEVEL
A1C refers to the percentage of glycosylation of the hemoglobin A1C chain and approximates average blood glucose levels over the previous two to three months from the slow turnover of red blood cells in the body.40 A1C was first included in the ADA guidelines as a diagnostic test for diabetes in 2010. Despite efforts to standardize laboratory tests, there are some limitations to A1C testing, and an incomplete correlation between A1C level and average glucose level in certain individuals (Table 341–43 ). For example, hemolytic anemias and acute blood loss can falsely lower A1C levels, whereas prior splenectomy and aplastic anemias, which increase erythrocyte age, can falsely elevate A1C levels. Hemoglobinopathies or hemoglobin variants can result in variable changes in A1C level and may be more prevalent among certain racial and ethnic groups.44–48 Point-of-care A1C measurements are not recommended for the diagnosis of diabetes.2,17 A1C testing should be performed in a laboratory using a method certified by the National Glycohemoglobin Standardization Program and consistent with the Diabetes Control and Complications Trial reference assay.
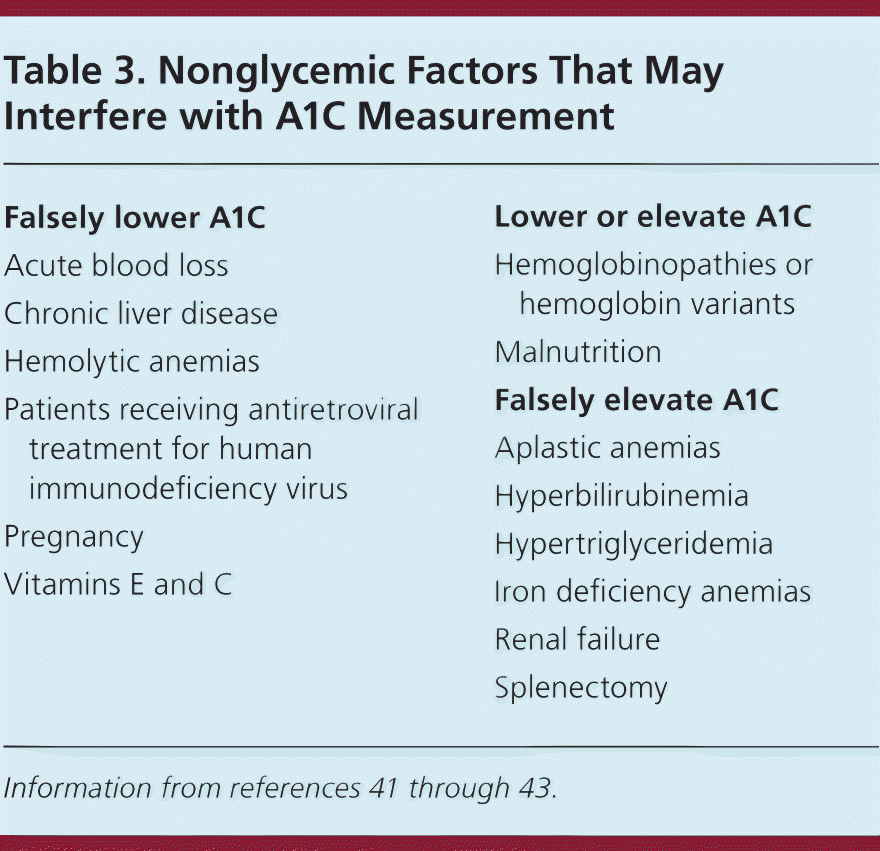
| Falsely lower A1C |
| Acute blood loss |
| Chronic liver disease |
| Hemolytic anemias |
| Patients receiving antiretroviral treatment for human immunodeficiency virus |
| Pregnancy |
| Vitamins E and C |
| Lower or elevate A1C |
| Hemoglobinopathies or hemoglobin variants |
| Malnutrition |
| Falsely elevate A1C |
| Aplastic anemias |
| Hyperbilirubinemia |
| Hypertriglyceridemia |
| Iron deficiency anemias |
| Renal failure |
| Splenectomy |
FASTING PLASMA GLUCOSE
The National Health and Nutrition Examination Survey data indicate that fasting plasma glucose values may identify as many as one-third more undiagnosed cases of diabetes compared with A1C levels.2,17,49 Fasting plasma glucose measurement should be obtained by a venous blood draw; elevated glucometer or continuous glucose monitor measurements are not considered diagnostic.17
SPECIAL TESTS
Increasingly, diabetes is being recognized as a spectrum of disorders including type 1 diabetes, type 2 diabetes, GDM, prediabetes, neonatal diabetes, maturity-onset diabetes of youth, and latent autoimmune diabetes in the adult. Overlap exists in the underlying etiology of these disorders.2,5,16,50–53 Autoimmune markers usually present in patients with type 1 diabetes include autoantibodies to one or more of the following: islet cells, insulin, glutamic acid decarboxylase, insulinoma-associated antigen-2, and zinc transporter (Table 417,50–53 ). Patients with idiopathic type 1 diabetes have no autoantibodies, and some patients with latent autoimmune diabetes in the adult or type 2 diabetes may have certain autoantibodies present making these tests less specific.5 Despite these concerns, the American Association of Clinical Endocrinologists recommend routine confirmation of type 1 diabetes using autoantibody testing.16 Additional research is required to determine whether further testing to classify the etiology of diabetes improves patient outcomes. In the meantime, additional testing is not routinely recommended.
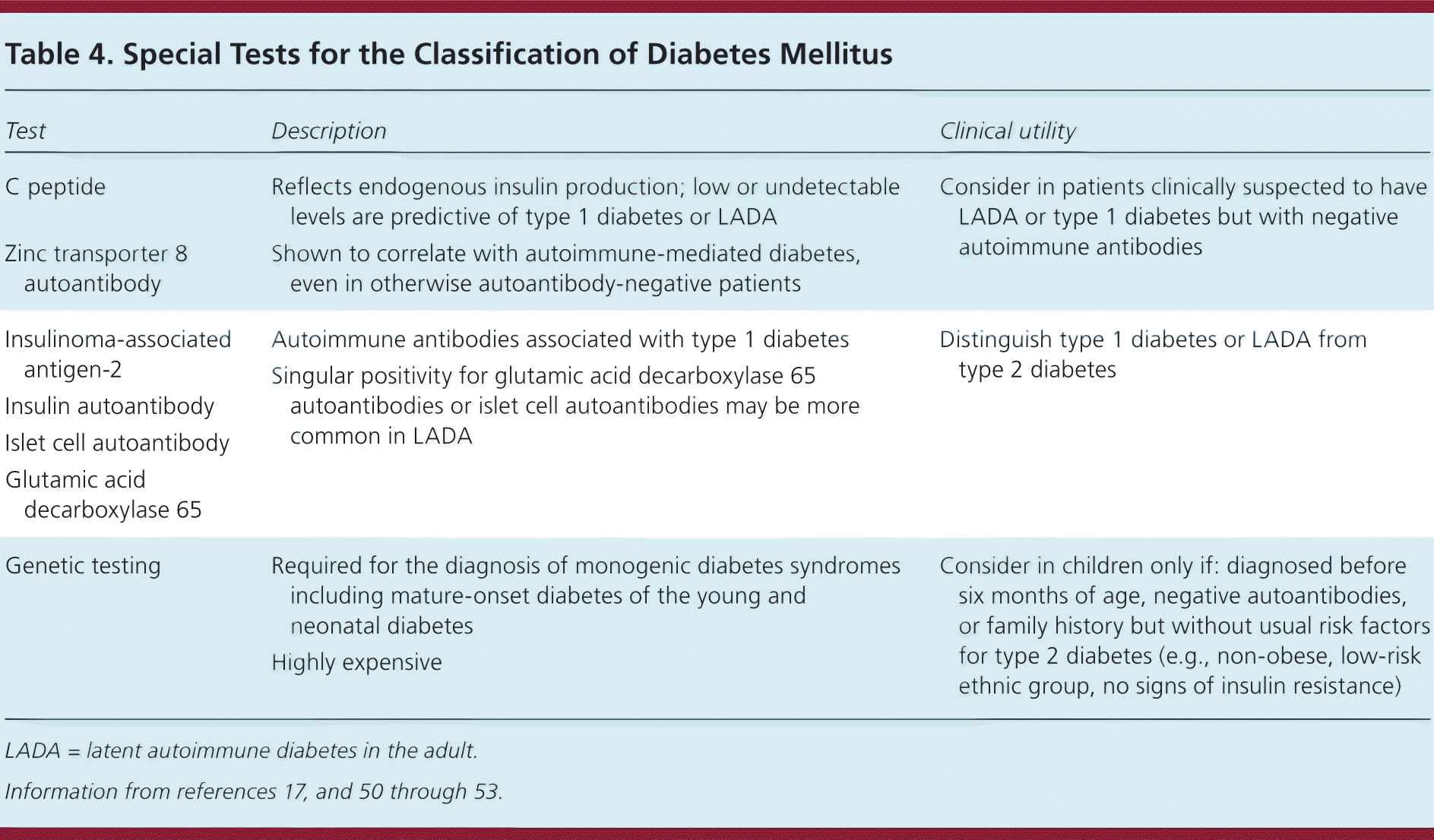
| Test | Description | Clinical utility |
|---|---|---|
| C peptide | Reflects endogenous insulin production; low or undetectable levels are predictive of type 1 diabetes or LADA | Consider in patients clinically suspected to have LADA or type 1 diabetes but with negative autoimmune antibodies |
| Zinc transporter 8 autoantibody | Shown to correlate with autoimmune-mediated diabetes, even in otherwise autoantibody-negative patients | |
| Insulinoma-associated antigen-2 | Autoimmune antibodies associated with type 1 diabetes | Distinguish type 1 diabetes or LADA from type 2 diabetes |
| Singular positivity for glutamic acid decarboxylase 65 autoantibodies or islet cell autoantibodies may be more common in LADA | ||
| Insulin autoantibody | ||
| Islet cell autoantibody | ||
| Glutamic acid decarboxylase 65 | ||
| Genetic testing | Required for the diagnosis of monogenic diabetes syndromes including mature-onset diabetes of the young and neonatal diabetes | Consider in children only if: diagnosed before six months of age, negative autoantibodies, or family history but without usual risk factors for type 2 diabetes (e.g., non-obese, low-risk ethnic group, no signs of insulin resistance) |
| Highly expensive |
Data Sources: A PubMed search was completed using the key terms diabetes mellitus, diabetes mellitus type 2, screening for diabetes mellitus, gestational diabetes, geriatrics, elderly, and pediatrics. The search included meta-analyses and reviews. Also searched were Essential Evidence Plus, the websites of the American Diabetes Association, the U.S. Preventive Services Task Force, the American Academy of Family Physicians, and the American Academy of Pediatrics. Search dates: March 2, 2015, and October 1, 2015.
The authors thank Karen Gunning, PharmD, for her editing and mentorship.
