
Am Fam Physician. 2016;93(3):online
Author disclosure: No relevant financial affiliations.

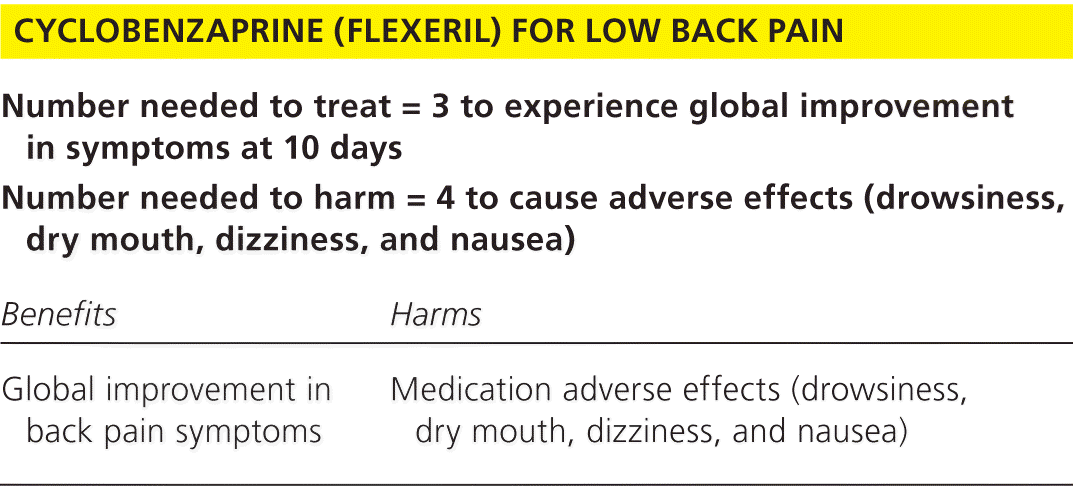
| Number needed to treat = 3 to experience global improvement in symptoms at 10 days | |
|---|---|
| Number needed to harm = 4 to cause adverse effects (drowsiness, dry mouth, dizziness, and nausea) | |
| Benefits | Harms |
| Global improvement in back pain symptoms | Medication adverse effects (drowsiness, dry mouth, dizziness, and nausea) |
Details For This Review
Study Population: Adults with uncomplicated low back pain
Efficacy End Points: Global improvement in symptoms by day 10 of treatment
Harm End Points: Drowsiness, dry mouth, dizziness, nausea
Narrative: The muscle relaxant cyclobenzaprine (Flexeril) is a common medication for low back pain that is used to reduce muscle spasm as an adjunct to pain control with nonnarcotic analgesics. Multiple reviews exist on this topic, including three we consider to be of relatively good quality, although with differing emphases. One is a Cochrane review that pools nonbenzodiazepine drugs together for analysis1; a second conducted for a joint clinical guideline from the American Pain Society and the American College of Physicians2; and the third is a comprehensive examination of cyclobenzaprine performed by an independent author group.3 This latter review, published in 2001, examined 14 publications (n = 2,440) comparing cyclobenzaprine with placebo, and was used as the basis for the joint clinical guideline; thus, we have concentrated on this study as our primary source for this summary.
In pooled analyses, treatment with cyclobenzaprine was more likely than placebo (odds ratio = 4.7; 95% confidence interval [CI], 2.7 to 8.1) to result in global improvement in symptoms by day 10 of treatment with a number needed to treat of 3 (95% CI, 2.0 to 4.5). Adverse effects, typically drowsiness, dry mouth, dizziness, and nausea, occurred in 53% of participants in the cyclobenzaprine group compared with 28% in the placebo group for a number needed to harm of 4.
Improvements in low back pain symptoms were present at all recorded time points, but the authors noted more of an effect size in the first four days of treatment. Because adverse effects would be present beyond the first few days, any optimum effectiveness of treatment would likely be short.
As with most trials of medication effectiveness, there was concern about publication bias. The authors used a file drawer test to show that the effect was unlikely because of publication bias alone.
Caveats: The general quality of included trials was low based on the Jadad scale. Moreover, there were critical failures in most of the trials, including lack of an intention-to-treat analysis for 11 of 13 trials.
One of the publications included in this review was available only in abstract form, and another was a difficult-to-access meta-analysis of 20 unpublished trials. These two publications constituted roughly 66% of participants in the review. Neither publication represented studies for which original peer-reviewed data were available.
It is also worth noting that the term “global improvement” is nebulously defined in many, if not all, of the included studies. Importantly, this outcome measure does not appear to include an integration of the adverse effects of cyclobenzaprine into the assessment of overall benefit.
There is a recent high-quality randomized controlled trial that suggests minimal pain-relieving effect of cyclobenzaprine when used in addition to naproxen for patients with acute low back pain.4 There may be some benefit, although of questionable magnitude, to the use of cyclobenzaprine. There is a high likelihood of adverse effects. Further research is needed to conclusively address this question. The possible benefit of questionable magnitude has led us to rate this topic yellow (unclear benefits).
Copyright 2016 The NNT Group (theNNT.com). Used with permission.
This series is coordinated by Dean A. Seehusen, MD, MPH, AFP Contributing Editor, and Daniel Runde, MD, from the NNT Group.
A collection of Medicine by the Numbers published in AFP is available at https://www.aafp.org/afp/mbtn.
The opinions and assertions contained herein are the private views of the authors and are not to be construed as official or as reflecting the views of the U.S. Army Medical Department or the U.S. Army at large.
This review is available from the NNT Group at http://www.thennt.com/nnt/cyclobenzaprine-treatment-low-back-pain/.
