
Am Fam Physician. 2016;93(3):203-210
Author disclosure: No relevant financial affiliations.
The advancing science of transplantation has led to more transplants and longer survival. As a result, primary care physicians are more involved in the care of transplant recipients. Immunosuppressive therapy has significantly decreased rates of transplant rejection but accounts for more than 50% of transplant-related deaths, often due to infections and other risks related to long-term use. Cardiovascular disease is the leading cause of non–transplant-related mortality. Aggressive risk factor management is recommended for transplant recipients, including a blood pressure goal of less than 130/80 mm Hg and statin therapy in kidney, liver, and heart recipients. Fertility typically increases posttransplant, and female transplant recipients should avoid pregnancy for one year after surgery. The best contraceptive choice is usually an intrauterine device. Because of the increased risk of infection, patients should be tested for graft dysfunction or infection if suspicion arises. Testing should be coordinated with the transplant center. Malignancies are a common cause of death in transplant recipients, requiring careful attention to screening recommendations and informed discussions with patients. Family physicians should maintain an ongoing relationship with the transplant team to discuss medication changes and the risk of infection or graft rejection.
Since 1988, more than 600,000 solid organ transplants have been performed in the United States, and approximately 130,000 patients are currently awaiting transplants.1 Five-year survival rates after liver and kidney transplants are 64% and 70%, respectively, and heart and lung recipients have a five-year survival rate of more than 50%.1 Because of the increasing number of surviving transplant recipients, care is gradually shifting to the primary care physician's office. This article focuses on the primary care of adults who have received solid organ transplants.
| Clinical recommendation | Evidence rating | References |
|---|---|---|
| A blood pressure goal of less than 130/80 mm Hg is recommended for all liver and kidney recipients. | C | 4, 6 |
| Statin therapy is recommended for all solid organ transplant recipients. | C | 4, 5, 8 |
| Pregnancy should be delayed for one year posttransplant. | C | 4, 5, 7, 19, 21 |
| Solid organ transplant recipients should be counseled about avoiding tobacco. | C | 4, 7 |
| Solid organ transplant recipients should be educated about sun avoidance and protection and evaluated by a dermatologist annually, starting within the first year posttransplant. | C | 36, 37 |
Medications
Immunosuppressive therapy accounts for more than 50% of transplant-related deaths, often due to infections and other risks related to long-term use.2 Patients typically receive high doses of two or more medications immediately after transplantation, with subsequent tapering over the following months to years to minimize long-term adverse effects.
Medication classes that reduce the risk of graft rejection include calcineurin inhibitors, which inhibit an enzyme that stimulates T lymphocytes; mammalian target of rapamycin inhibitors, which impede cell growth and proliferation; and purine synthesis inhibitors, which include azathioprine (Imuran), methotrexate, and the commonly used subclass of mycophenolic acid derivatives, which inhibit enzymes needed for the growth of T and B lymphocytes. Corticosteroids are often also used early after transplantation, but are tapered as quickly as possible.3 Most of the preferred immunosuppressive medications are metabolized through the cytochrome P450 3A4 pathway and can interact with other medications. Because transplant recipients receive multiple medications, primary care physicians should be mindful of polypharmacy when starting new drugs. Table 1 reviews these medications, including adverse effects and examples of significant drug-drug interactions.4,5 Because of the extensive list of interactions, particularly with calcineurin inhibitors, changes to any medication should be discussed with the transplant team.
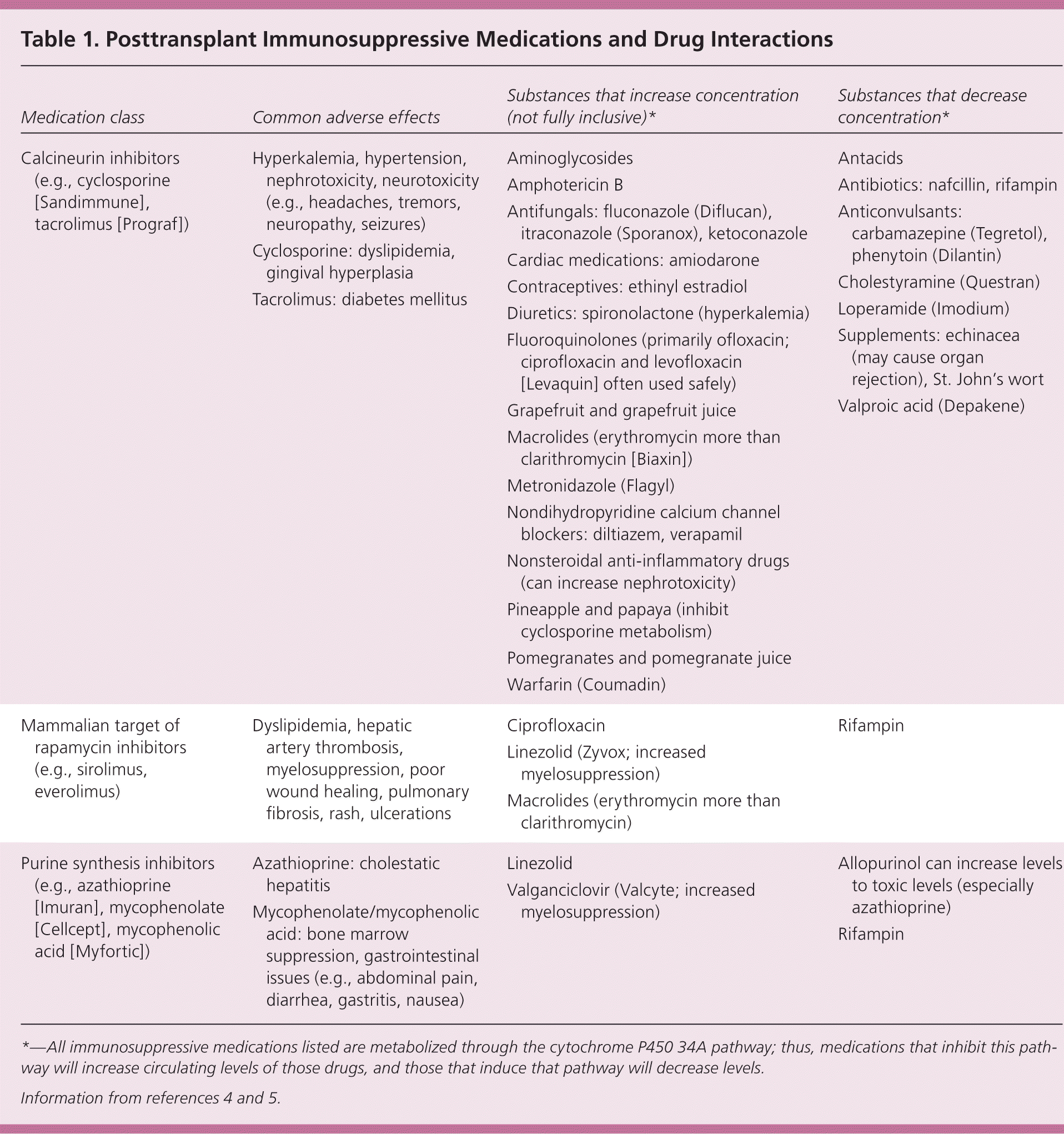
| Medication class | Common adverse effects | Substances that increase concentration (not fully inclusive)* | Substances that decrease concentration* |
|---|---|---|---|
|
|
|
|
|
|
|
|
|
|
|
|
Posttransplant Disease Management
Cardiovascular disease and renal failure are the leading causes of posttransplant morbidity and mortality independent of graft rejection.4 Increased cardiovascular risk is thought to be mediated by increased rates of hypertension, diabetes mellitus, hyperlipidemia, and obesity. Table 2 summarizes risk factors and provides recommendations for screening and treatment of posttransplant systemic diseases.4–8
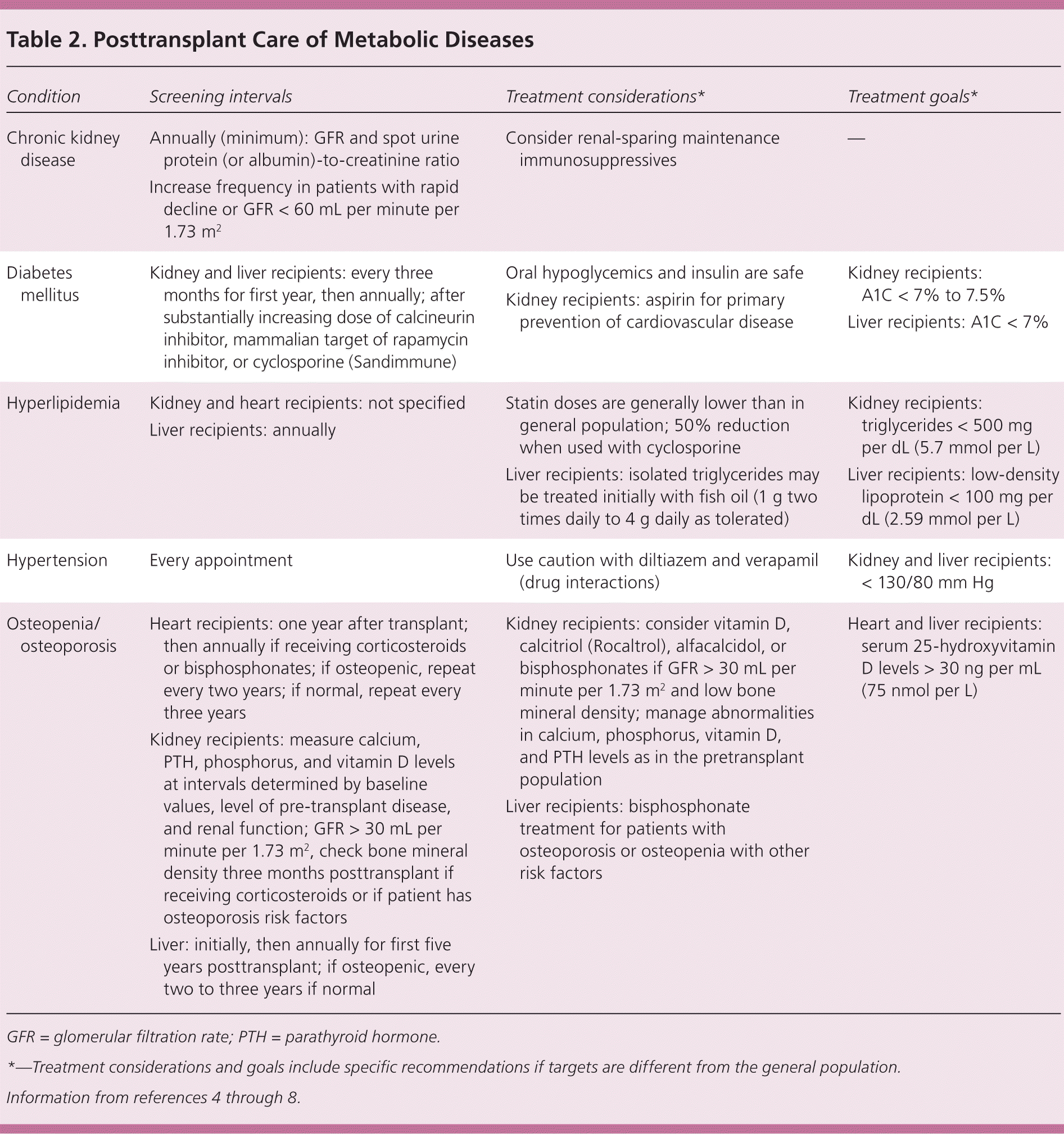
| Condition | Screening intervals | Treatment considerations* | Treatment goals* |
|---|---|---|---|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
HYPERTENSION
The Eighth Joint National Committee did not specifically address the target blood pressure range for solid organ transplant recipients.9 The goal suggested by transplant experts is less than 130/80 mm Hg.4,6 The choice of antihypertensive therapy depends on patient comorbidities and the immunosuppressive regimen. There is only limited evidence supporting the use of specific antihypertensive classes, and transplant experts do not strongly recommend any class for first-line therapy.
Calcium channel blockers (CCBs) may counteract the vasoconstrictive effects of calcineurin inhibitors to control blood pressure and reduce nephrotoxicity. A Cochrane review found 17 randomized trials comparing CCBs with placebo or no treatment in 1,255 patients.10 Based on low-quality evidence, it concluded that CCB use may reduce the risk of graft loss (68 vs. 90 per 1,000 over a median of one year) but has no effect on overall mortality or acute graft rejection. Dihydropyridine CCBs are less likely to interact with calcineurin inhibitors, and the Cochrane review concluded that they may have a more favorable effect on serum creatinine levels and glomerular filtration rate.10 However, nondihydropyridine CCBs may be used if therapeutic levels of calcineurin inhibitors are monitored.4,6,10 Use of a nondihydropyridine CCB can reduce dosage requirements and therefore the cost of calcineurin inhibitors.6,7 Angiotensin-converting enzyme inhibitors and angiotensin receptor blockers are first-line agents in kidney recipients if urinary protein excretion exceeds 1 g per day, but their use can cause increased serum creatinine levels, hyperkalemia, and anemia.7 Thiazide diuretics are recommended in kidney recipients to counteract the sodium-related effects of calcineurin inhibitors. However, loop and thiazide diuretics should be used with caution in liver recipients.4,7 Kidney recipients with resistant hypertension should be referred back to the transplant center to rule out allograft vascular compromise and complications related to the native kidneys, which are not typically removed during transplant surgery.7
HYPERLIPIDEMIA
Statins are the preferred agent to treat hyperlipidemia and reduce cardiovascular risk in solid organ transplant recipients. The American Association for the Study of Liver Disease/American Society of Transplantation recommends a low-density lipoprotein goal of less than 100 mg per dL (2.59 mmol per L) in liver recipients.4 The Kidney Disease Improving Global Outcomes and International Society of Heart and Lung Transplantation guidelines recommend universal statin therapy in kidney and heart recipients.5,8 Initial statin doses in all solid organ transplant recipients are lower than those in the general population because of potential interactions with calcineurin inhibitors.5,8
All of the recommendations above are based on expert opinion. A 2014 Cochrane review of 22 studies with 3,465 patients found that statin therapy had inconsistent or uncertain effects on all-cause and cardiovascular mortality, major cardiovascular events, and fatal or nonfatal myocardial infarctions in kidney recipients.11 Evidence favoring statin treatment is more robust in heart recipients; statin therapy in first-time recipients reduces rates of mortality and allograft rejection with hemodynamic compromise at one year,12 reduces cardiac allograft vasculopathy, and improves long-term outcomes regardless of lipid levels.5
CHRONIC KIDNEY DISEASE
Non-kidney solid organ transplant recipients have higher posttransplant rates of chronic kidney disease. Strategies recommended to reduce disease progression mirror those in the general population: blood pressure and glucose control, and use of medications that block the renin-angiotensin-aldosterone system. Additionally, transplant centers may decrease calcineurin inhibitor doses, potentially transitioning to an inhibitor-free regimen to preserve renal function.4,5
DIABETES
Diabetes can be preexisting or can occur for the first time posttransplant. All major transplant societies recommend screening and treatment, in accordance with guidelines from the American Diabetes Association.4,5,7 The target A1C is less than 7% in most transplant recipients.4,5,7 Insulin is typically required, but oral hypoglycemic agents can also be used with the same restrictions for comorbid conditions as in the general population.4,7 The use of thiazolidinediones has not been proven safe in liver recipients.4 The risk of new-onset diabetes is highest in the first three months after transplantation. Although there is no consistent evidence that new-onset diabetes resolves as immunosuppressive therapy is tapered, glycemic control often improves with time.4,5,7
OBESITY
Obesity (body mass index greater than 30 kg per m2) occurs in roughly 20% to 30% of liver and kidney recipients who were not obese before the transplant.4,13 Improved appetite after transplantation and medication adverse effects are likely contributing factors.4 Lifestyle modifications remain the cornerstone of therapy, but bariatric surgery is not contraindicated.4,7
BONE DISEASE
Osteopenia and osteoporosis are common before transplantation. Multiple factors, including corticosteroid therapy, cause bone mineral density to decrease and fracture risk to increase in the immediate posttransplant period.14,15 Although transplant experts use standard definitions for osteoporosis and osteopenia based on bone mineral density, fracture risk in solid organ transplant recipients does not correlate directly with these values.5,15,16
Evidence regarding fracture risk reduction with any agent is inconsistent.15,17 Kidney recipients can have abnormal serum levels of phosphorus, parathyroid hormone, and vitamin D. Treatment for these conditions should be similar to that in the pretransplant period. In kidney recipients with osteoporosis, bisphosphonate therapy is recommended only in the first year after the transplant. Bone biopsy should be considered in kidney recipients because of their increased risk of adynamic bone disease, which is worsened by bisphosphonate use.15 Heart recipients should receive bisphosphonates for the first year after transplant to prevent bone loss.5 Bisphosphonate therapy is also recommended for liver recipients who have osteoporosis or osteopenia with risk factors.4
Graft Dysfunction
Graft dysfunction is often first noted through routine screening tests or surveillance biopsies. Screening for rejection or other graft complications is site-specific and based on individual patient characteristics, including time from transplant and stability of previous testing.4 The transplant team should be notified immediately if there is any concern for graft dysfunction or rejection. Table 3 presents typical screening tests according to transplant type.4,5,7,18
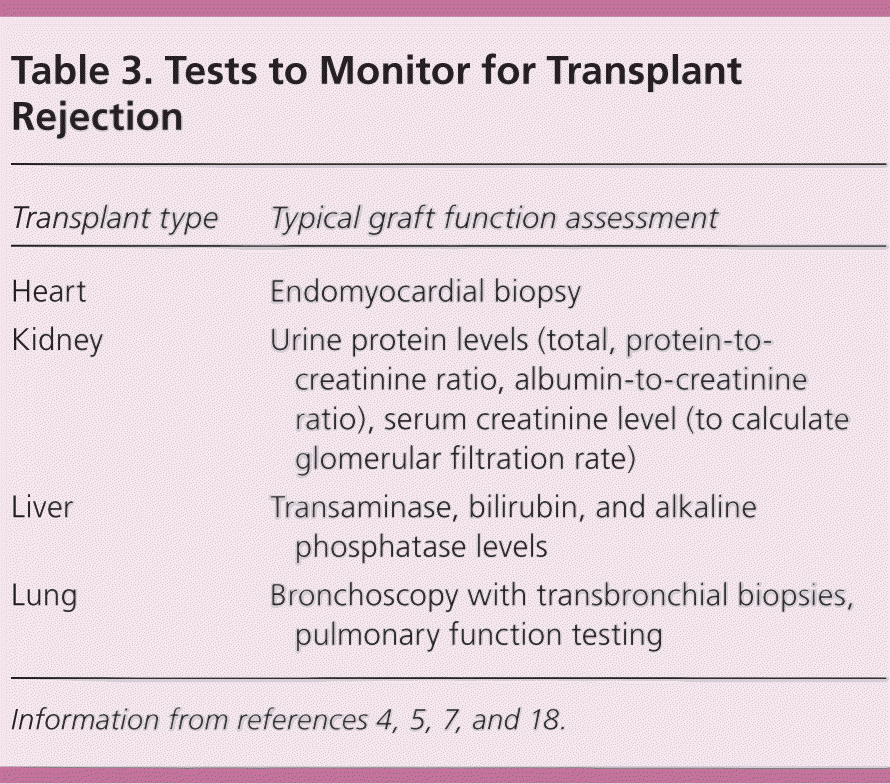
| Transplant type | Typical graft function assessment |
|---|---|
| Heart | Endomyocardial biopsy |
| Kidney | Urine protein levels (total, protein-to-creatinine ratio, albumin-to-creatinine ratio), serum creatinine level (to calculate glomerular filtration rate) |
| Liver | Transaminase, bilirubin, and alkaline phosphatase levels |
| Lung | Bronchoscopy with transbronchial biopsies, pulmonary function testing |
Reproductive and Sexual Health
Transplant recipients often struggle with infertility, erectile dysfunction, and other reproductive and sexual concerns before and after the transplant. Fertility generally improves post-transplant.19,20 The National Transplantation Pregnancy Registry follows pregnancy outcomes, and patients are encouraged to register; enrollment forms are available at http://www.ntpr.giftoflifeinstitute.org.
Male transplant recipients often have incomplete recovery of gonadal function, but are still able to father a child. Erectile dysfunction can be treated with phosphodiesterase-5 inhibitors.4,5,7 The risk of pregnancy complications is not increased when the father is a kidney recipient. Men may wish to bank sperm before they use mammalian target of rapamycin inhibitors.7
Pregnancy during the 12 months following transplantation is associated with an increased risk of preterm delivery and graft dysfunction, rejection, or loss.19,21 Reliable contraception should be used before the transplant because ovulation can occur as early as one month posttransplant. The use of an intrauterine device avoids interactions with medications, does not increase the risk of infection, and is not affected by typical immunosuppressive therapies. For these reasons, physicians should encourage female transplant recipients to strongly consider an intrauterine device for contraception.20,21 The medical eligibility criteria for contraceptive use from the Centers for Disease Control and Prevention include specific recommendations for contraceptive types during the first two years after solid organ transplantation.22 A comparison of contraceptive methods specific to transplant recipients is presented in Table 4.19–21
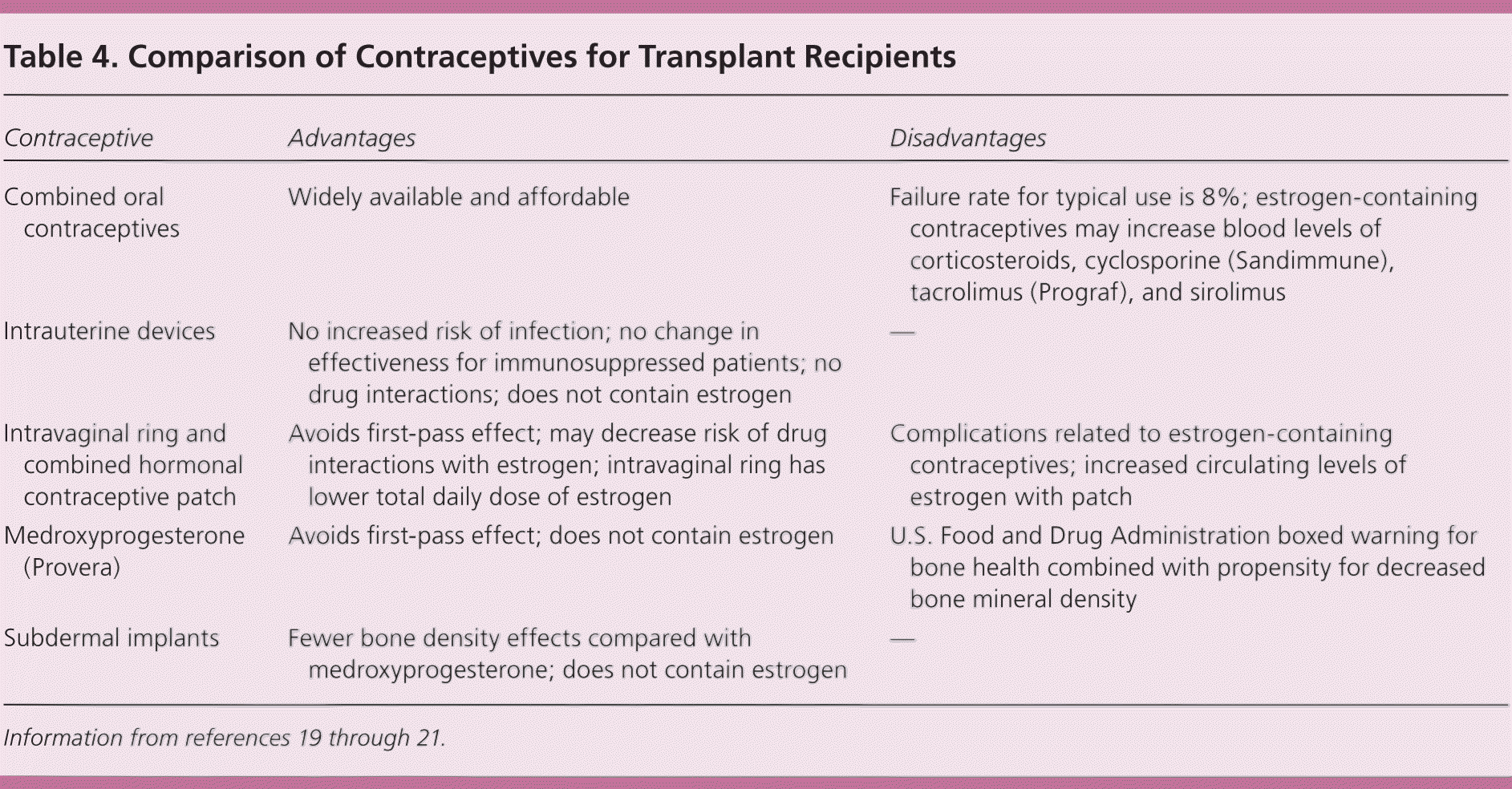
| Contraceptive | Advantages | Disadvantages |
|---|---|---|
| Combined oral contraceptives | Widely available and affordable | Failure rate for typical use is 8%; estrogen-containing contraceptives may increase blood levels of corticosteroids, cyclosporine (Sandimmune), tacrolimus (Prograf), and sirolimus |
| Intrauterine devices | No increased risk of infection; no change in effectiveness for immunosuppressed patients; no drug interactions; does not contain estrogen | — |
| Intravaginal ring and combined hormonal contraceptive patch | Avoids first-pass effect; may decrease risk of drug interactions with estrogen; intravaginal ring has lower total daily dose of estrogen | Complications related to estrogen-containing contraceptives; increased circulating levels of estrogen with patch |
| Medroxyprogesterone (Provera) | Avoids first-pass effect; does not contain estrogen | U.S. Food and Drug Administration boxed warning for bone health combined with propensity for decreased bone mineral density |
| Subdermal implants | Fewer bone density effects compared with medroxyprogesterone; does not contain estrogen | — |
Transplant centers should be notified before attempts to conceive; this allows for evaluation of graft function and adjustment of immunosuppressive regimens, if needed. Ideally, patients should not have experienced graft rejection within the past year; should have stable graft function, no acute infections, and controlled comorbidities; and should be on a stable immunosuppressive regimen before conceiving.4,7,19 Certain immunosuppressive agents should be discontinued before pregnancy because of the increased risk of fetal compromise. Mycophenolic acid compounds have about a 25% risk of birth defects, and mammalian target of rapamycin inhibitors increase the risk of preterm delivery, decreased fetal weight, and delayed skeletal ossification.7,19,23 Azathioprine, cyclosporine (Sandimmune), and tacrolimus (Prograf) have not been shown to increase the risk of birth defects.19
Transplant recipients have a high risk of miscarriage, gestational hypertension, preeclampsia, gestational diabetes, and fetal complications such as prematurity and low birth weight. They typically require consultation or management by obstetricians and maternal-fetal medicine subspecialists. Although cesarean deliveries are not routinely recommended, they are performed more often in kidney (44%) and liver (57%) recipients.19
Most transplant recipients may safely breastfeed their infants. The benefits of breastfeeding while receiving corticosteroids, cyclosporine, tacrolimus, or azathioprine outweigh the risks of drug exposure. Physicians should monitor infant serum drug levels for the first few weeks of life, and should recommend discontinuing breastfeeding only if measurable levels are detected. Breastfeeding is not recommended for mothers receiving mycophenolic acid (Myfortic), sirolimus, everolimus, or belatacept (Nulojix); these agents have limited safety data, and mycophenolic acid is known to increase the risk of fetal malformations from in utero exposure.23
Infection
Immunocompromised patients have an increased vulnerability to common and opportunistic pathogens. Typical treatment and prophylactic regimens for opportunistic infections in transplant recipients are summarized in Table 5.4,5,7 Treatment plans are best determined in consultation with the transplant center.
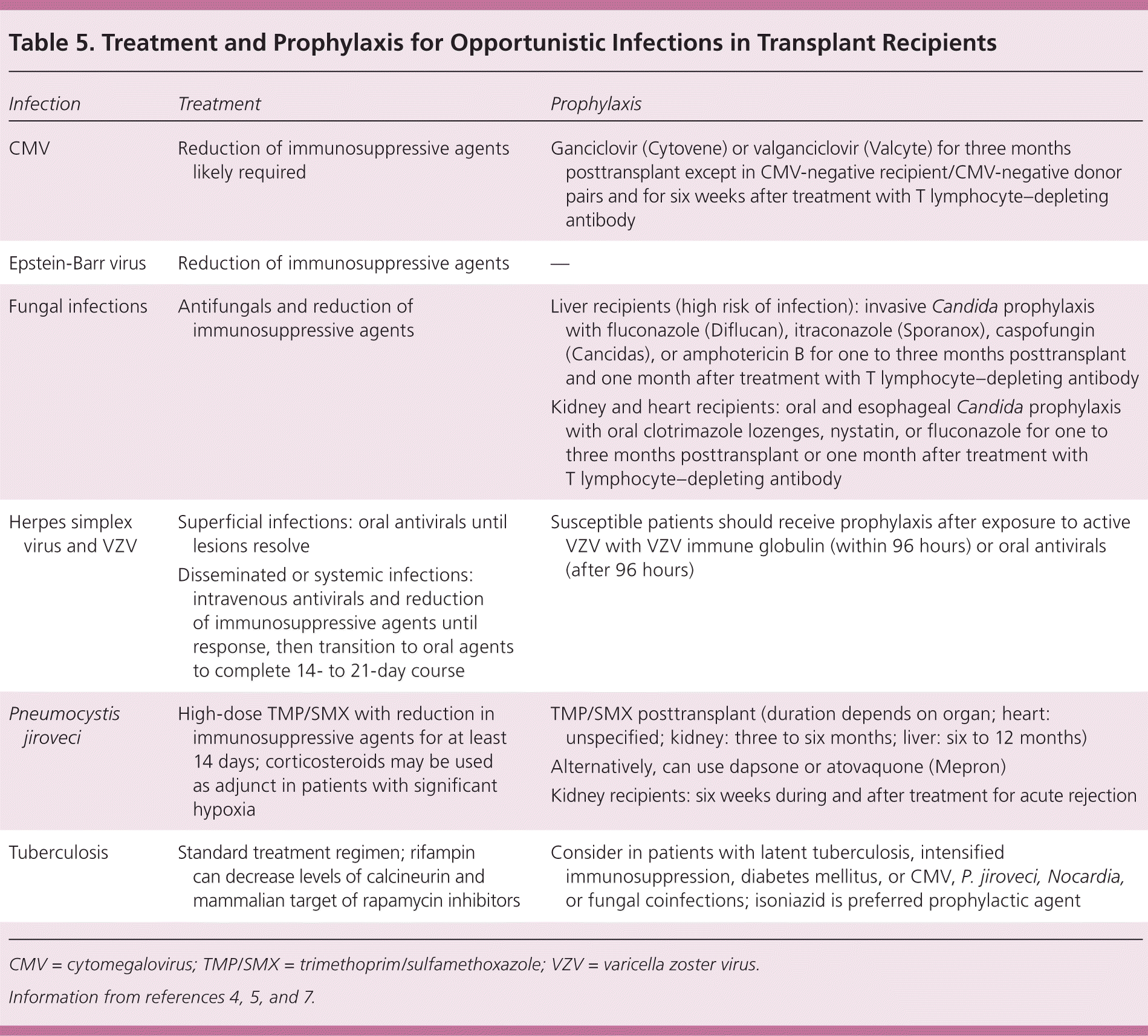
| Infection | Treatment | Prophylaxis |
|---|---|---|
| CMV | Reduction of immunosuppressive agents likely required | Ganciclovir (Cytovene) or valganciclovir (Valcyte) for three months posttransplant except in CMV-negative recipient/CMV-negative donor pairs and for six weeks after treatment with T lymphocyte–depleting antibody |
| Epstein-Barr virus | Reduction of immunosuppressive agents | — |
| Fungal infections | Antifungals and reduction of immunosuppressive agents | Liver recipients (high risk of infection): invasive Candida prophylaxis with fluconazole (Diflucan), itraconazole (Sporanox), caspofungin (Cancidas), or amphotericin B for one to three months posttransplant and one month after treatment with T lymphocyte–depleting antibody |
| Kidney and heart recipients: oral and esophageal Candida prophylaxis with oral clotrimazole lozenges, nystatin, or fluconazole for one to three months posttransplant or one month after treatment with T lymphocyte–depleting antibody | ||
| Herpes simplex virus and VZV | Superficial infections: oral antivirals until lesions resolve | Susceptible patients should receive prophylaxis after exposure to active VZV with VZV immune globulin (within 96 hours) or oral antivirals (after 96 hours) |
| Disseminated or systemic infections: intravenous antivirals and reduction of immunosuppressive agents until response, then transition to oral agents to complete 14- to 21-day course | ||
| Pneumocystis jiroveci | High-dose TMP/SMX with reduction in immunosuppressive agents for at least 14 days; corticosteroids may be used as adjunct in patients with significant hypoxia | TMP/SMX posttransplant (duration depends on organ; heart: unspecified; kidney: three to six months; liver: six to 12 months) |
| Alternatively, can use dapsone or atovaquone (Mepron) | ||
| Kidney recipients: six weeks during and after treatment for acute rejection | ||
| Tuberculosis | Standard treatment regimen; rifampin can decrease levels of calcineurin and mammalian target of rapamycin inhibitors | Consider in patients with latent tuberculosis, intensified immunosuppression, diabetes mellitus, or CMV, P. jiroveci, Nocardia, or fungal coinfections; isoniazid is preferred prophylactic agent |
Preventive Care
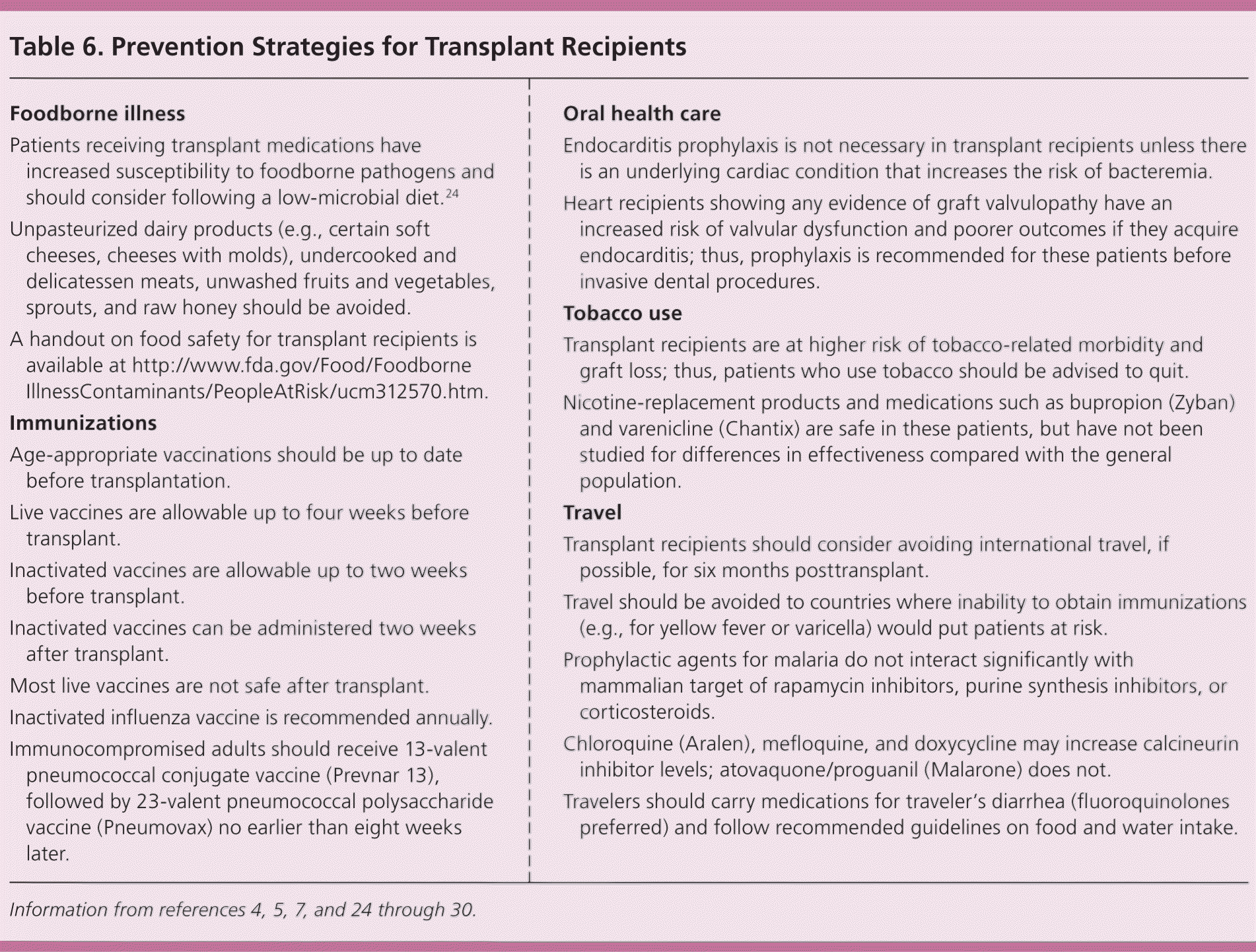
| Foodborne illness |
| Patients receiving transplant medications have increased susceptibility to foodborne pathogens and should consider following a low-microbial diet.24 |
| Unpasteurized dairy products (e.g., certain soft cheeses, cheeses with molds), undercooked and delicatessen meats, unwashed fruits and vegetables, sprouts, and raw honey should be avoided. |
| A handout on food safety for transplant recipients is available at http://www.fda.gov/Food/FoodborneIllnessContaminants/PeopleAtRisk/ucm312570.htm. |
| Immunizations |
| Age-appropriate vaccinations should be up to date before transplantation. |
| Live vaccines are allowable up to four weeks before transplant. |
| Inactivated vaccines are allowable up to two weeks before transplant. |
| Inactivated vaccines can be administered two weeks after transplant. |
| Most live vaccines are not safe after transplant. |
| Inactivated influenza vaccine is recommended annually. |
| Immunocompromised adults should receive 13-valent pneumococcal conjugate vaccine (Prevnar 13), followed by 23-valent pneumococcal polysaccharide vaccine (Pneumovax) no earlier than eight weeks later. |
| Oral health care |
| Endocarditis prophylaxis is not necessary in transplant recipients unless there is an underlying cardiac condition that increases the risk of bacteremia. |
| Heart recipients showing any evidence of graft valvulopathy have an increased risk of valvular dysfunction and poorer outcomes if they acquire endocarditis; thus, prophylaxis is recommended for these patients before invasive dental procedures. |
| Tobacco use |
| Transplant recipients are at higher risk of tobacco-related morbidity and graft loss; thus, patients who use tobacco should be advised to quit. |
| Nicotine-replacement products and medications such as bupropion (Zyban) and varenicline (Chantix) are safe in these patients, but have not been studied for differences in effectiveness compared with the general population. |
| Travel |
| Transplant recipients should consider avoiding international travel, if possible, for six months posttransplant. |
| Travel should be avoided to countries where inability to obtain immunizations (e.g., for yellow fever or varicella) would put patients at risk. |
| Prophylactic agents for malaria do not interact significantly with mammalian target of rapamycin inhibitors, purine synthesis inhibitors, or corticosteroids. |
| Chloroquine (Aralen), mefloquine, and doxycycline may increase calcineurin inhibitor levels; atovaquone/proguanil (Malarone) does not. |
| Travelers should carry medications for traveler's diarrhea (fluoroquinolones preferred) and follow recommended guidelines on food and water intake. |
CANCER SCREENING
Malignancy rates are higher in all transplant recipients.24 The incidence of the most common cancers—colon, lung, breast, and prostate—are twofold higher than that in the general population.31 The risk of other cancers, including Kaposi sarcoma, non-Hodgkin lymphoma, nonmelanomatous skin cancers, and cancers related to viral infections, is increased as much as 20-fold.31,32 Survival rates among transplant recipients who develop secondary cancers are often less than 10% over five years.33 Most of the currently accepted guidelines for the general population do not specifically address cancer screening in transplant recipients.34,35
Screening recommendations for breast, prostate, and colon cancers in transplant recipients mirror guidelines from the U.S. Preventive Services Task Force.34,35 Because cervical cancer is mediated by an oncogenic virus, some experts have debated more frequent surveillance, and the American Cancer Society recommends that physicians consider this on a case-by-case basis; however, there is no rigorous evidence to support this practice.34,35 Physicians should consider the patient's comorbidities and life expectancy, and have informed discussions with their patients.33
Nonmelanomatous squamous cell and basal cell skin cancers are the most common malignancies in transplant recipients. Squamous cell cancers tend to develop at a younger age, are typically more aggressive, and metastasize more often.32,36 The incidence in the United States and western Europe increases from 10% to 27% at 10 years posttransplant to 40% to 60% at 20 years.36 The National Comprehensive Cancer Network recommends aggressive treatment, even for precancerous lesions such as actinic keratosis, and a low threshold for biopsy in any suspicious lesion.37 Patients should be referred to a dermatologist within one year of the transplant and should be screened for skin cancer at least annually. Physicians should counsel patients to avoid sun exposure and to mitigate risk with appropriate clothing and sunscreen.37
Data Sources: We conducted an Ovid search of all publications with the keywords solid organ transplantation, primary care, immunizations, screening, pregnancy, contraception, and rejection. In addition, we searched the Cochrane database, the National Guideline Clearinghouse, PubMed, and Clinical Queries. Most of our resources are meta-analyses, evidence-based guidelines, and subtopic-specific review articles. Search dates: May 2012, September and October 2014, and March and October 2015.
The opinions and assertions contained herein are the private views of the authors and are not to be construed as official or as reflecting the views of the U.S. Navy, Department of Defense, or the U.S. government.
