
Am Fam Physician. 2016;93(6):506-508
Summary of Recommendations and Evidence
The USPSTF recommends screening for major depressive disorder (MDD) in adolescents aged 12 to 18 years. Screening should be implemented with adequate systems in place to ensure accurate diagnosis, effective treatment, and appropriate follow-up (Table 1). B recommendation.
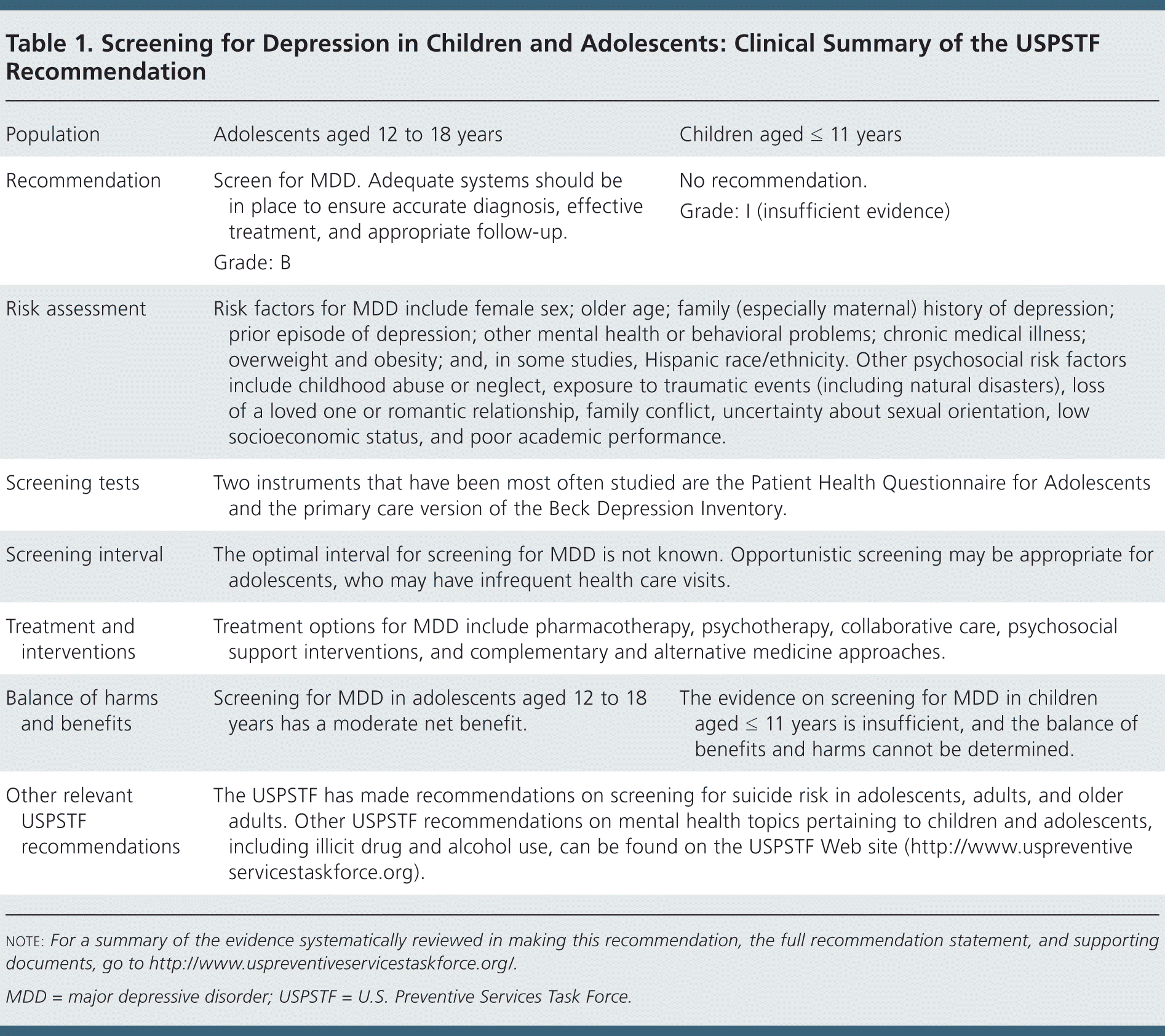
| Population | Adolescents aged 12 to 18 years | Children aged ≤ 11 years |
| Recommendation | Screen for MDD. Adequate systems should be in place to ensure accurate diagnosis, effective treatment, and appropriate follow-up. | No recommendation. |
| Grade: B | Grade: I (insufficient evidence) | |
| Risk assessment | Risk factors for MDD include female sex; older age; family (especially maternal) history of depression; prior episode of depression; other mental health or behavioral problems; chronic medical illness; overweight and obesity; and, in some studies, Hispanic race/ethnicity. Other psychosocial risk factors include childhood abuse or neglect, exposure to traumatic events (including natural disasters), loss of a loved one or romantic relationship, family conflict, uncertainty about sexual orientation, low socioeconomic status, and poor academic performance. | |
| Screening tests | Two instruments that have been most often studied are the Patient Health Questionnaire for Adolescents and the primary care version of the Beck Depression Inventory. | |
| Screening interval | The optimal interval for screening for MDD is not known. Opportunistic screening may be appropriate for adolescents, who may have infrequent health care visits. | |
| Treatment and interventions | Treatment options for MDD include pharmacotherapy, psychotherapy, collaborative care, psychosocial support interventions, and complementary and alternative medicine approaches. | |
| Balance of harms and benefits | Screening for MDD in adolescents aged 12 to 18 years has a moderate net benefit. | The evidence on screening for MDD in children aged ≤ 11 years is insufficient, and the balance of benefits and harms cannot be determined. |
| Other relevant USPSTF recommendations | The USPSTF has made recommendations on screening for suicide risk in adolescents, adults, and older adults. Other USPSTF recommendations on mental health topics pertaining to children and adolescents, including illicit drug and alcohol use, can be found on the USPSTF Web site (http://www.uspreventiveservicestaskforce.org). | |
The USPSTF concludes that the current evidence is insufficient to assess the balance of benefits and harms of screening for MDD in children aged 11 years or younger. I statement.
Rationale
IMPORTANCE
Depression is a leading cause of disability in the United States. Children and adolescents with MDD typically have functional impairments in their performance at school or work, as well as in their interactions with their families and peers. Depression can also negatively affect the developmental trajectories of affected youth. MDD in children and adolescents is strongly associated with recurrent depression in adulthood; other mental disorders; and increased risk for suicidal ideation, suicide attempts, and suicide completion.
In nationally representative U.S. surveys, about 8% of adolescents reported having major depression in the past year. Little is known about the prevalence of MDD in children. Among children and adolescents aged 8 to 15 years, 2% of males and 4% of females reported having MDD in the past year.
DETECTION
The USPSTF found adequate evidence that screening instruments for depression can accurately identify MDD in adolescents aged 12 to 18 years in primary care settings. The USPSTF found no studies of screening instruments for depression in children aged 11 years or younger in primary care (or comparable) settings and concluded that the evidence is inadequate.
BENEFITS OF EARLY DETECTION AND INTERVENTION AND TREATMENT
The USPSTF found no studies that directly evaluated whether screening for MDD in adolescents in primary care (or comparable) settings leads to improved health and other outcomes. However, the USPSTF found adequate evidence that treatment of MDD detected through screening in adolescents is associated with moderate benefit (for example, improved depression severity, depression symptoms, or global functioning scores).
The USPSTF found no studies that directly evaluated whether screening for MDD in children aged 11 years or younger in primary care (or comparable) settings leads to improved health and other outcomes and found inadequate evidence on the benefits of treatment in children with screen-detected MDD.
HARMS OF EARLY DETECTION AND INTERVENTION AND TREATMENT
The USPSTF found no direct evidence on the harms of screening for MDD in adolescents. Medications for the treatment of depression, such as selective serotonin reuptake inhibitors (SSRIs), have known harms. However, the magnitude of the harms of pharmacotherapy is small if patients are closely monitored, as recommended by the U.S. Food and Drug Administration (FDA). The USPSTF found adequate evidence on the harms of psychotherapy and psychosocial support in adolescents and estimates that the magnitude of these harms is small to none.
The USPSTF found inadequate evidence on the harms of screening for or treatment of MDD in children aged 11 years or younger.
USPSTF ASSESSMENT
The USPSTF concludes with moderate certainty that screening for MDD in adolescents aged 12 to 18 years has a moderate net benefit.
The USPSTF concludes that the evidence on screening for MDD in children aged 11 years or younger is insufficient. Evidence is lacking, and the balance of benefits and harms cannot be determined.
Clinical Considerations
PATIENT POPULATION UNDER CONSIDERATION
This recommendation applies to children and adolescents aged 18 years or younger who do not have a diagnosis of MDD. This recommendation focuses on screening for MDD and does not address screening for other depressive disorders, such as minor depression or dysthymia.
ASSESSMENT OF RISK
The USPSTF recommends screening for MDD in all adolescents but notes that several risk factors might help identify patients who are at higher risk. The causes of MDD are not fully known and likely involve a combination of genetic, biological, and environmental factors. Risk factors for MDD in children and adolescents include female sex; older age; family (especially maternal) history of depression; prior episode of depression; other mental health or behavioral problems; chronic medical illness; overweight and obesity; and, in some studies, Hispanic race/ethnicity. Other psychosocial risk factors include childhood abuse or neglect, exposure to traumatic events (including natural disasters), loss of a loved one or romantic relationship, family conflict, uncertainty about sexual orientation, low socioeconomic status, and poor academic performance.
SCREENING TESTS
Many MDD screening instruments have been developed for use in primary care and have been used in adolescents. Two that have been most often studied are the Patient Health Questionnaire for Adolescents and the primary care version of the Beck Depression Inventory. Data on the accuracy of MDD screening instruments in younger children are limited.
SCREENING INTERVALS
The USPSTF found no evidence on appropriate or recommended screening intervals, and the optimal interval is unknown. Repeated screening may be most productive in adolescents with risk factors for MDD. Opportunistic screening may be appropriate for adolescents, who may have infrequent health care visits.
TREATMENT OR INTERVENTIONS
Treatment options for MDD in children and adolescents include pharmacotherapy, psychotherapy, collaborative care, psychosocial support interventions, and complementary and alternative medicine approaches. Fluoxetine is approved by the FDA for treatment of MDD in children aged 8 years or older, and escitalopram is approved for treatment of MDD in adolescents aged 12 to 17 years. The FDA has issued a boxed warning for antidepressants, recommending that patients of all ages who start antidepressant therapy be monitored appropriately and observed closely for clinical worsening, suicidality, or unusual changes in behavior.1 Collaborative care is a multicomponent, health care system–level intervention that uses care managers to link primary care providers, patients, and mental health specialists.
SUGGESTIONS FOR PRACTICE REGARDING THE I STATEMENT
In deciding whether to screen for MDD in children aged 11 years or younger, primary care providers should consider the following issues.
Potential Preventable Burden. Little is known about the prevalence of MDD in children aged 11 years or younger. The mean age of onset of MDD is about 14 to 15 years. Early onset is associated with worse outcomes. The average duration of a depressive episode in childhood varies widely, from 2 to 17 months.
Potential Harms. The USPSTF found inadequate evidence on the harms of screening for MDD in children. The USPSTF concluded that screening itself is unlikely to be associated with significant harms, aside from opportunity costs, labeling and potential stigma associated with a positive result, and referral for further evaluation and treatment.
The USPSTF concluded, on the basis of a previous review, that the use of SSRIs in children is associated with harms, specifically risk for suicidality. Evidence on the harms of psychotherapy alone or in combination with SSRIs in children is limited. Newer studies provide little additional evidence on treatment harms in children and adolescents but do not suggest more risks. Only 4 studies examined the harms of treatment with SSRIs in children and adolescents. These studies found no increased risk for suicidality associated with antidepressant use, but risk for rare events could not be precisely determined because the studies had limited statistical power. No trials of psychotherapy or combined interventions in children examined harms.
Current Practice. The USPSTF found no evidence on the current frequency of or methods used in primary care for screening for MDD in children.
ADDITIONAL APPROACHES TO PREVENTION
The Community Preventive Services Task Force recommends collaborative care for the management of depressive disorders, based on strong evidence of effectiveness in improving depression symptoms, adherence and response to treatment, and remission and recovery from depression. For this and related recommendations from the Community Preventive Services Task Force, go to http://www.thecommunityguide.org/mentalhealth/index.html.
USEFUL RESOURCES
In a separate recommendation statement, the USPSTF concluded that the current evidence is insufficient to assess the balance of benefits and harms of screening for suicide risk in primary care settings, including among adolescents (I statement). Other USPSTF recommendations on mental health topics pertaining to children and adolescents, including illicit drug and alcohol use, can be found on the USPSTF Web site (http://www.uspreventiveservicestaskforce.org).
This recommendation statement was published online ahead of print in Ann Intern Med. February 9, 2016, and in Pediatrics. February 9, 2016.
The “Other Considerations,” “Discussion,” “Update of Previous USPSTF Recommendation,” and “Recommendations of Others” sections of this recommendation statement are available at http://www.uspreventiveservicestaskforce.org/Page/Document/UpdateSummaryFinal/depression-in-children-and-adolescents-screening1.
The USPSTF recommendations are independent of the U.S. government. They do not represent the views of the Agency for Healthcare Research and Quality, the U.S. Department of Health and Human Services, or the U.S. Public Health Service.
