
A more recent article on peripartum depression is available.
Am Fam Physician. 2016;93(10):852-858
Related editorial: Opportunities to Detect and Manage Perinatal Depression in Men.
Patient information: See related handout on depression during and after pregnancy, written by the authors of this article.
Author disclosure: No relevant financial affiliations.
Peripartum depression affects up to one in seven women and is associated with significant maternal and neonatal morbidity if untreated. A history of depression is the strongest risk factor for developing peripartum depression. The U.S. Preventive Services Task Force recommends screening pregnant and postpartum women for depression. Both two-step and one-step screening strategies are effective in identifying peripartum depression. Peripartum depression should be distinguished from the baby blues, which is characterized by short duration, mild symptoms, and minimal impact on functioning. Women with peripartum depression should be evaluated for bipolar disorder, postpartum psychosis, and suicidal risk. For first-time mothers, adolescent mothers, and mothers who have experienced a traumatic delivery, home health visits, telephone-based peer support, and psychotherapy may help prevent peripartum depression. Mild to moderate depression should be treated with psychotherapy or selective serotonin reuptake inhibitors, whereas moderate to severe depression should be treated with a combination of psychotherapy and medication. Citalopram, escitalopram, and sertraline appear to be the safest selective serotonin reuptake inhibitors during pregnancy, whereas fluvoxamine, paroxetine, and sertraline are preferred in breastfeeding women because they lead to the lowest serum medication levels in breastfed infants. Patients with psychosis, active suicidal thoughts, or thoughts of harming their newborns should receive same-day psychiatric consultation and referral for possible inpatient treatment.
Major or minor depression during or immediately after pregnancy occurs in up to one in seven women.1 Peripartum depression is associated with significant neonatal morbidity, such as failure to thrive, attachment disorder, and developmental delay at one year of age2; treatment improves functioning in these children.3 Peripartum depression is also associated with significant maternal morbidity, including decreased energy, poor concentration,4 sleep disturbances,5 and disruption of maternal-infant bonding and healthy family dynamics. Maternal suicide is a more common cause of peripartum mortality than postpartum hemorrhage or hypertensive disorders.6 Family physicians are in a vital position to identify and treat patients with peripartum depression.
WHAT IS NEW ON THIS TOPIC: PERIPARTUM DEPRESSION
First-time mothers, adolescent mothers, and mothers who have experienced a traumatic delivery may benefit from home health visits, telephone-based peer support, and psychotherapy to prevent peripartum depression.
| Clinical recommendation | Evidence rating | References |
|---|---|---|
| Women may be screened for peripartum depression using a single tool, such as the Edinburgh Postnatal Depression Scale, or using a two-step process, such as the Patient Health Questionnaire-2 followed by a more comprehensive screening test if the initial screen is positive. | C | 18–20 |
| All women should be screened for depression during pregnancy and the postpartum period. | B | 27, 28 |
| Women with peripartum depression should be evaluated for bipolar disorder, postpartum psychosis, and suicide risk and referred for emergent psychiatric evaluation when appropriate. | C | 29, 30, 64 |
| There is insufficient evidence to recommend selective serotonin reuptake inhibitors, estrogen, or docosahexaenoic acid for the prevention of peripartum depression. | C | 37, 45, 46 |
| There is insufficient evidence to recommend one selective serotonin reuptake inhibitor over another in the treatment of moderate to severe postpartum depression. | C | 55 |
Etiology and Risk Factors
The etiology of peripartum depression is unclear. There are a number of theories, including abnormalities of maternal neurotransmitters,7 decreased levels of estrogen,8 dysfunction of the hypothalamic-pituitary-adrenal axis,9 thyroid dysfunction,10 and genetic predisposition.11 Biologic, social, genetic, and psychological factors likely contribute to varying degrees. Practically speaking, the etiology does not affect diagnosis and treatment.
A 2010 meta-analysis of 57 studies and a 2014 population-based analysis demonstrated several risk factors for peripartum depression, including fear of childbirth (odds ratio [OR] = 3.8), tobacco use (OR = 3.25), adolescence (OR = 3.14), single status (not married or living with a partner; OR = 2.86), lower socioeconomic status (OR = 2.59), and age of 40 years or older (OR = 1.41).12,13 Other risk factors include domestic violence (OR = 3.1),14 maternal anxiety (OR = 2.7),15 unintended pregnancy (OR = 1.41),16 and gestational diabetes mellitus (OR = 2.29).17 The greatest risk factor is a history of depression (OR = 29).12 However, no identifiable risk factors may be present.
Screening
The Diagnostic and Statistical Manual of Mental Disorders, 5th ed. (DSM-5) classifies peripartum depression as a major depressive disorder that is identified during pregnancy or within four weeks postpartum,5 although some experts extend this to within one year postpartum.18 The DSM-5 criteria for major depressive disorder are listed in Table 1.5

| A. Five (or more) of the following symptoms have been present during the same 2-week period and represent a change from previous functioning; at least one of the symptoms is either (1) depressed mood or (2) loss of interest or pleasure. | |
| Note: Do not include symptoms that are clearly attributable to another medical condition. | |
| 1. Depressed mood most of the day, nearly every day, as indicated by either subjective report (e.g., feels sad, empty, hopeless) or observation made by others (e.g., appears tearful). (Note: In children and adolescents, can be irritable mood.) | |
| 2. Markedly diminished interest or pleasure in all, or almost all, activities most of the day, nearly every day (as indicated by either subjective account or observation). | |
| 3. Significant weight loss when not dieting or weight gain (e.g., a change of more than 5% of body weight in a month), or decrease or increase in appetite nearly every day. (Note: In children, consider failure to make expected weight gain.) | |
| 4. Insomnia or hypersomnia nearly every day. | |
| 5. Psychomotor agitation or retardation nearly every day (observable by others, not merely subjective feelings of restlessness or being slowed down). | |
| 6. Fatigue or loss of energy nearly every day. | |
| 7. Feelings of worthlessness or excessive or inappropriate guilt (which may be delusional) nearly every day (not merely self-reproach or guilt about being sick). | |
| 8. Diminished ability to think or concentrate, or indecisiveness, nearly every day (either by subjective account or as observed by others). | |
| 9. Recurrent thoughts of death (not just fear of dying), recurrent suicidal ideation without a specific plan, or a suicide attempt or a specific plan for committing suicide. | |
| B. The symptoms cause clinically significant distress or impairment in social, occupational, or other important areas of functioning. | |
| C. The episode is not attributable to the physiological effects of a substance or to another medical condition. | |
| Note: Criteria A-C represent a major depressive episode. | |
| Note: Responses to a significant loss (e.g., bereavement, financial ruin, losses from a natural disaster, a serious medical illness or disability) may include the feelings of intense sadness, rumination about the loss, insomnia, poor appetite, and weight loss noted in Criterion A, which may resemble a depressive episode. Although such symptoms may be understandable or considered appropriate to the loss, the presence of a major depressive episode in addition to the normal response to a significant loss should also be carefully considered. This decision inevitably requires the exercise of clinical judgment based on the individual's history and the cultural norms for the expression of distress in the context of loss. | |
| D. The occurrence of the major depressive episode is not better explained by schizoaffective disorder, schizophrenia, schizophreniform disorder, delusional disorder, or other specified and unspecified schizophrenia spectrum and other psychotic disorders. | |
| E. There has never been a manic episode or a hypomanic episode. Note: This exclusion does not apply if all of the manic-like or hypomanic-like episodes are substance-induced or are attributable to the physiological effects of another medical condition. | |
Because many symptoms of major depressive disorder are not specific for peripartum depression, validated screening tests should be used to evaluate pregnant women (Table 219,20 ). However, because of limitations in study size and design, a preferred screening test for peripartum depression has not been identified.21
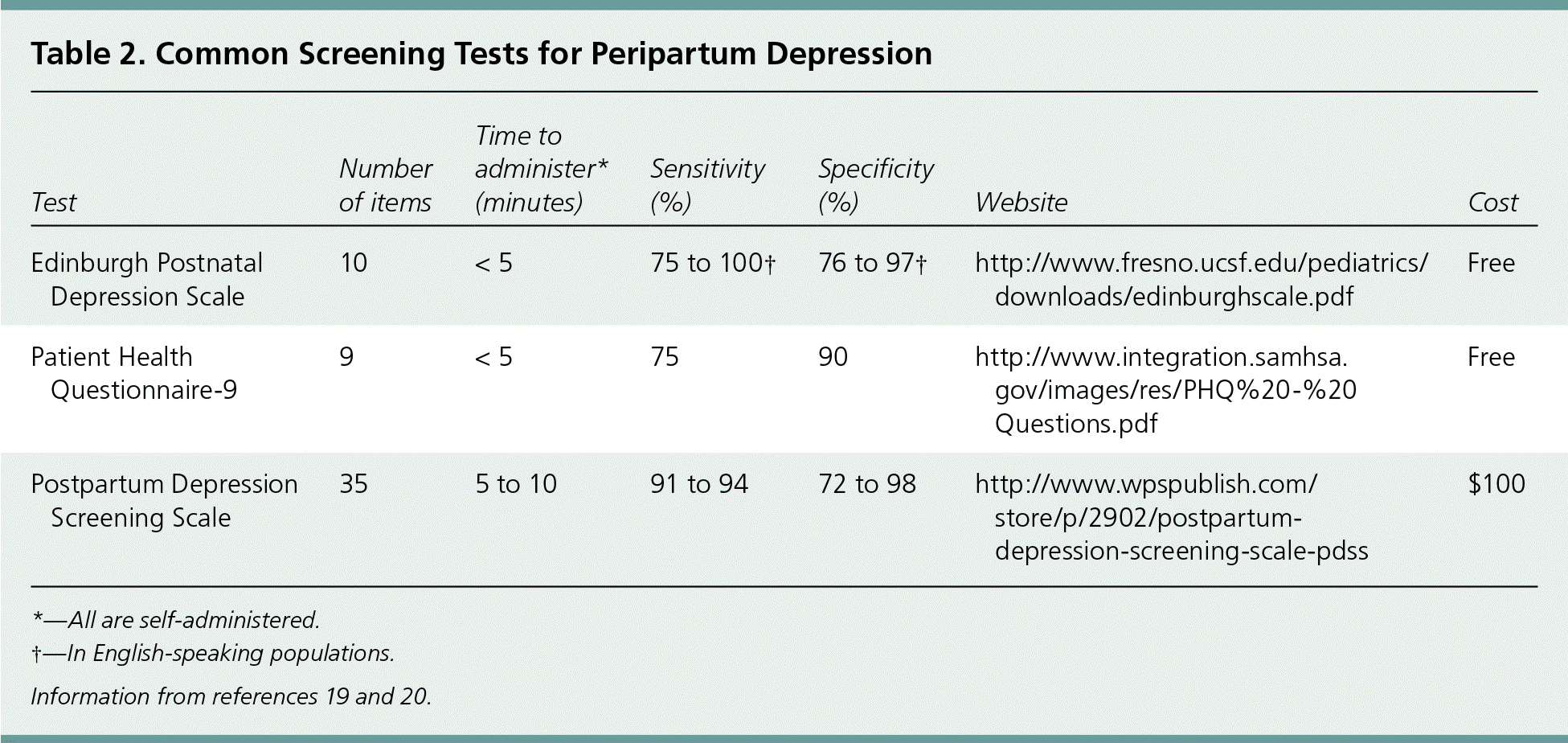
| Test | Number of items | Time to administer* (minutes) | Sensitivity (%) | Specificity (%) | Website | Cost |
|---|---|---|---|---|---|---|
| Edinburgh Postnatal Depression Scale | 10 | < 5 | 75 to 100† | 76 to 97† | http://www.fresno.ucsf.edu/pediatrics/downloads/edinburghscale.pdf | Free |
| Patient Health Questionnaire-9 | 9 | < 5 | 75 | 90 | http://www.integration.samhsa.gov/images/res/PHQ%20-%20Questions.pdf | Free |
| Postpartum Depression Screening Scale | 35 | 5 to 10 | 91 to 94 | 72 to 98 | http://www.wpspublish.com/store/p/2902/postpartum-depression-screening-scale-pdss | $100 |
Both one-step and two-step screening strategies are effective in identifying peripartum depression. The 10-item Edinburgh Postnatal Depression Scale22 (available at https://www.aafp.org/afp/2010/1015/p926.html#afp20101015p926-f1) has a sensitivity of 75% to 100% and a specificity of 76% to 97% in English-speaking populations.19 By comparison, the Patient Health Questionnaire-9 (available at https://www.aafp.org/afp/2012/0115/p139.html#afp20120115p139-t4) has a sensitivity of 75% and a specificity of 90%, and the 35-question Postpartum Depression Screening Scale has a sensitivity of 91% to 94% and a specificity of 72% to 98%.20 The Patient Health Questionnaire-9 and the Postpartum Depression Screening Scale can be used to classify the severity of peripartum depression.
Many family physicians are familiar with two-step screening for major depressive disorder, which uses a shorter screening test initially, such as the Patient Health Questionnaire-2 (available at https://www.aafp.org/afp/2012/0115/p139.html#afp20120115p139-t3), followed by a more comprehensive screening test if either of the two questions is positive. This approach is also feasible in screening for peripartum depression.23
Women with symptoms such as sleep disturbance, anxiety, or irritability who do not meet the criteria for peripartum depression may have the baby blues. Symptoms of the baby blues usually develop during the first few days after delivery and resolve spontaneously within 10 days.24 There is no evidence that the baby blues increase the risk of developing peripartum depression.25
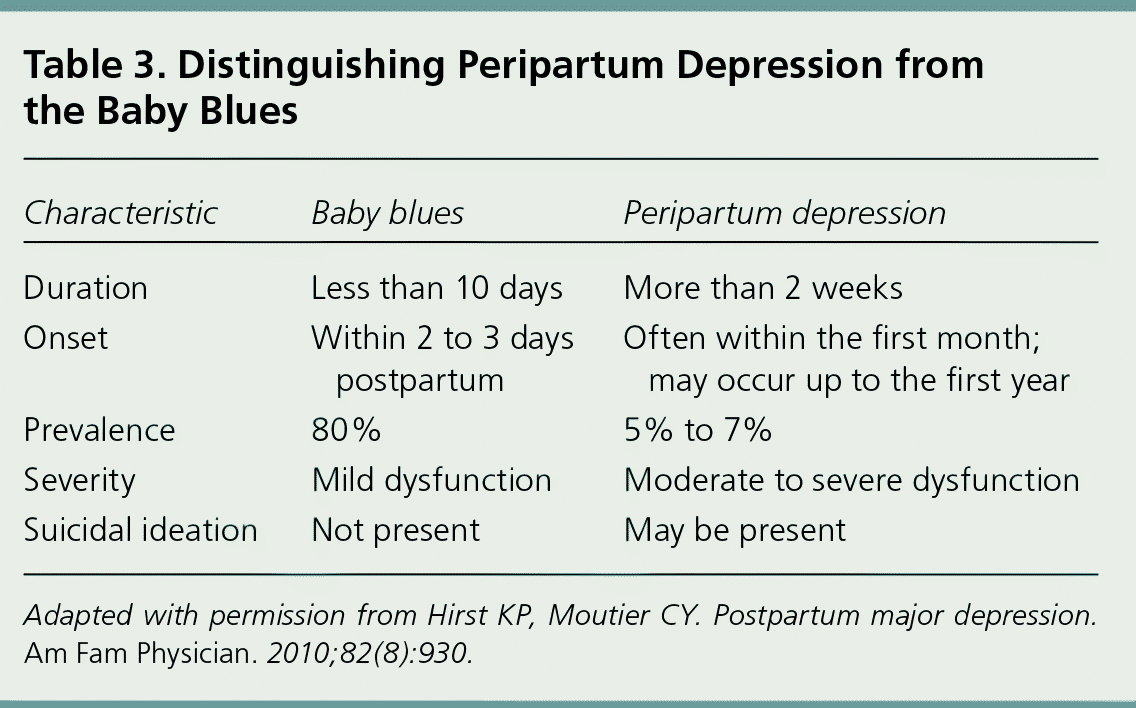
| Characteristic | Baby blues | Peripartum depression |
|---|---|---|
| Duration | Less than 10 days | More than 2 weeks |
| Onset | Within 2 to 3 days postpartum | Often within the first month; may occur up to the first year |
| Prevalence | 80% | 5% to 7% |
| Severity | Mild dysfunction | Moderate to severe dysfunction |
| Suicidal ideation | Not present | May be present |
A statement from the U.S. Preventive Services Task Force recommends screening adults 18 years and older, including pregnant and postpartum women, for depression, with adequate systems in place to ensure accurate diagnosis, effective treatment, and appropriate follow-up.27 The American College of Obstetricians and Gynecologists (ACOG) states that although definitive evidence of benefit is lacking, clinicians should screen patients for depression and anxiety at least once during the perinatal period using a standardized, validated tool.20 The American Academy of Pediatrics (AAP) Mental Health Task Force recommends incorporating screening for peripartum depression into the prenatal visit and into the well-child care schedule at one, two, four, and six months.2 The 2014 National Institute for Health and Care Excellence guideline states that screening for depression should be considered as part of a general discussion about mental health and well-being during pregnancy and the early postnatal period.28
Evaluation
When peripartum depression is suspected, a stepwise approach should be taken initially. Patients should be asked about a personal history of bipolar disorder or episodes of mania or hypomania; if present, the self-reported Mood Disorder Questionnaire can be used to screen for bipolar disorder.29 Psychiatric evaluation is recommended if the Mood Disorder Questionnaire result is positive. Next, patients should be asked about the presence of cognitive disturbances (“Do you ever see or hear things that other people do not?”), delusions, or disorganized behavior (including rambling or pressured speech). These symptoms suggest postpartum psychosis, which is a psychiatric emergency.30 Finally, all women with peripartum depression should be assessed for suicidal ideation, both active (“Have you ever thought about hurting yourself?”) and passive (“Have you ever wished you would go to sleep and not wake up?”), as well as homicidal ideation. Suicidal or homicidal ideation warrants emergent psychiatric evaluation.
Prevention
Studies support the use of certain preventive measures, particularly in first-time mothers, adolescent mothers, and mothers who have experienced a traumatic delivery.31–35 Interventions with good-quality evidence are home health visits, telephone-based peer support, and psychotherapy (cognitive behavior and interpersonal therapies).36 Mothers who experienced a traumatic delivery had the greatest improvement in symptom scores with these therapies.31 Intensive prenatal and postpartum education programs showed benefit in younger mothers, particularly primiparous adolescents, with significant improvements in Edinburgh Postnatal Depression Scale scores.31–34
A 2005 Cochrane review concluded that although the use of selective serotonin reuptake inhibitors (SSRIs) shows a modest benefit in the prevention of peripartum depression for mothers with previously diagnosed peripartum depression, there are insufficient data to recommend this use.37 There are safety concerns regarding SSRI use during pregnancy. Although causality has not been established, SSRI use during pregnancy is associated with increased risk of persistent pulmonary hypertension of the newborn, lower Apgar scores, attention-deficit/hyperactivity disorder, and speech delay.38–42 A 2015 Bayesian analysis showed no association between the use of sertraline (Zoloft), citalopram (Celexa), and escitalopram (Lexapro) in pregnancy and severe birth defects; however, paroxetine (Paxil) and fluoxetine (Prozac) use was associated with a small increase in specific birth defects.43 A large cohort study showed an increased risk of autism spectrum disorders in the 22 children of mothers who took SSRIs during the second or third trimester of pregnancy (adjusted hazard ratio = 2.17).44
There is insufficient evidence to support the use of estrogen for the prevention or treatment of peripartum depression.45 Similarly, evidence does not support the use of dietary supplements, such as docosahexaenoic acid46; herbal therapy, such as St. John's wort 47; or hypnosis48 for prevention of peripartum depression.
Nonpharmacologic Therapy
Psychotherapy alone is considered first-line treatment for mild to moderate peripartum depression, whereas psychotherapy is often combined with medication in patients with severe symptoms.49 Cognitive behavior therapy has the most evidence supporting its effectiveness.50,51 Partner participation in psychotherapy may also lead to improved symptom scores.52
Evidence is equivocal regarding the use of exercise to treat peripartum depression,53 and hypnosis has no demonstrated benefit.48 The specialized training of visiting nurses in the recognition of peripartum depression and subsequent counseling has been shown to be more beneficial than using untrained health care visitors.54
In patients with severe peripartum depression that is refractory to medication or who have contraindications to medication use, electroconvulsive therapy is effective.49
Pharmacologic Treatment
SSRIs are the cornerstone of moderate to severe peripartum depression treatment.55 In a randomized controlled trial comparing antidepressants with community-based psychosocial intervention for peripartum depression, SSRIs were superior, with a number needed to treat of 4 at four weeks.56 Although no substantial evidence supports the use of one SSRI over another, there are a few points to consider when selecting an agent.55,57 If the patient has a history of response to a particular SSRI, it is reasonable to use that medication initially.57 Postpartum women can be sensitive to medications because of hormone effects on liver enzymes, increased volume of distribution, and increased levels of drug-binding proteins; therefore, some experts recommend starting a medication at one-half of the regular dose and titrating slowly.57 In contrast, pregnant women often require higher doses of medications because of larger volumes of distribution.58 If the patient is breastfeeding, it is reasonable to consider the relative transfer of a medication into breast milk.59,60
CONSIDERATIONS FOR BREASTFEEDING WOMEN
A 2008 ACOG guideline provides levels of lactation risk for various antidepressant medications, corresponding to data previously established regarding infant serum medication levels while breastfeeding.61 A 2013 AAP clinical report supports this disease-oriented risk stratification.62 Moderately safe medications in the ACOG guideline correspond to infant serum medication levels that reach at least 10% of maternal serum levels. Of note, although the ACOG guideline lists fluvoxamine, nortriptyline (Pamelor), and sertraline in the safer category, the AAP places them in the next higher risk category because of serum medication levels. Table 4 lists the ACOG and AAP risk categories of commonly prescribed antidepressants.62 Regardless of risk level, parents and clinicians should be attentive to symptoms of antidepressant toxicity in the infant, such as lethargy, irritability unresponsive to normal comfort measures, and impaired feeding.63
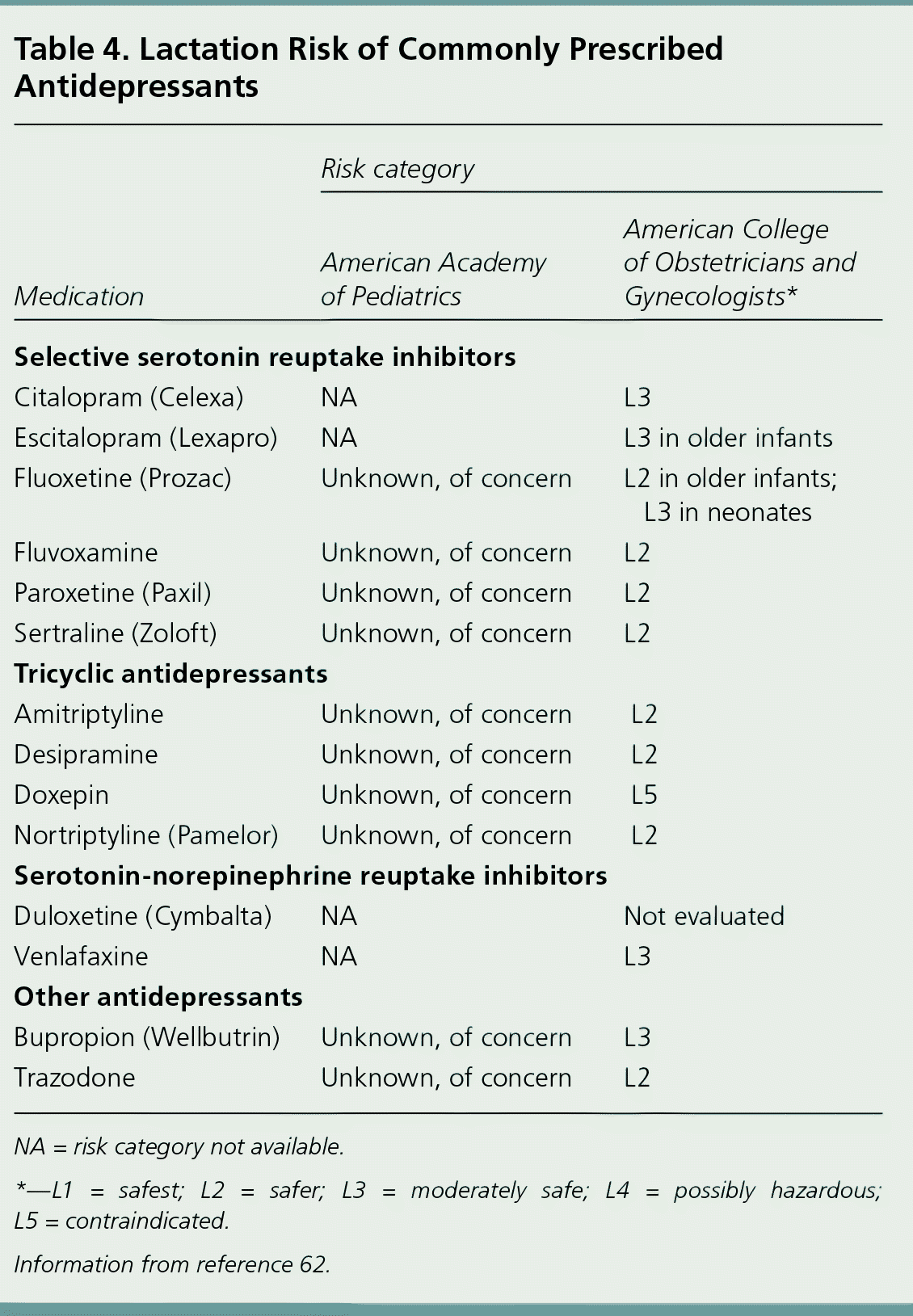
| Medication | Risk category | |
|---|---|---|
| American Academy of Pediatrics | American College of Obstetricians and Gynecologists* | |
| Selective serotonin reuptake inhibitors | ||
| Citalopram (Celexa) | NA | L3 |
| Escitalopram (Lexapro) | NA | L3 in older infants |
| Fluoxetine (Prozac) | Unknown, of concern | L2 in older infants; L3 in neonates |
| Fluvoxamine | Unknown, of concern | L2 |
| Paroxetine (Paxil) | Unknown, of concern | L2 |
| Sertraline (Zoloft) | Unknown, of concern | L2 |
| Tricyclic antidepressants | ||
| Amitriptyline | Unknown, of concern | L2 |
| Desipramine | Unknown, of concern | L2 |
| Doxepin | Unknown, of concern | L5 |
| Nortriptyline (Pamelor) | Unknown, of concern | L2 |
| Serotonin-norepinephrine reuptake inhibitors | ||
| Duloxetine (Cymbalta) | NA | Not evaluated |
| Venlafaxine | NA | L3 |
| Other antidepressants | ||
| Bupropion (Wellbutrin) | Unknown, of concern | L3 |
| Trazodone | Unknown, of concern | L2 |
FOLLOW-UP
If pharmacotherapy is used for peripartum depression, patients should be reassessed in one to two weeks either in person or by telephone. Treatment response should be evaluated after four to six weeks and medication doses titrated if remission has not occurred. Although no studies specifically address the management of resistant peripartum depression, following an approach established for major depressive disorder is advised. This may include switching to a different medication within the same class, adding a second antidepressant from a different class, adding an adjunctive medication such as an antipsychotic, or starting electroconvulsive therapy.64,65 Patients should be treated for a minimum of six months, even if they achieve remission.57
Emergency Treatment
Patients with active suicidal thoughts, thoughts of harming their newborns, or psychosis should have same-day psychiatric consultation for possible inpatient treatment.64,65 Antipsychotics are the treatment of choice for women with psychosis during pregnancy or the postpartum period, despite a lack of high-quality evidence to support their effectiveness.30,66
Data Sources: A PubMed search was completed in Clinical Queries using the key terms peripartum depression, screening, prevention, and treatment. The search included meta-analyses, randomized controlled trials, clinical trials, and reviews. Also searched were the Agency for Healthcare Research and Quality evidence reports, Clinical Evidence, the Cochrane database, Essential Evidence, the Institute for Clinical Systems Improvement, the National Guideline Clearinghouse database, and the U.S. Preventive Services Task Force. Search dates: November 1, 2015, and February 5, 2016.
