Am Fam Physician. 2016;94(2):161-166
Author disclosure: No relevant financial affiliations.
Liraglutide (Saxenda) is a glucagon-like peptide-1 (GLP-1) receptor agonist that, in addition to stimulating insulin release and inhibiting glucagon secretion, slows gastric emptying and increases satiety after eating. It is labeled as an adjunct to diet and exercise for weight management in overweight or obese adults. It is a higher-dose version of the same product used to treat type 2 diabetes mellitus.
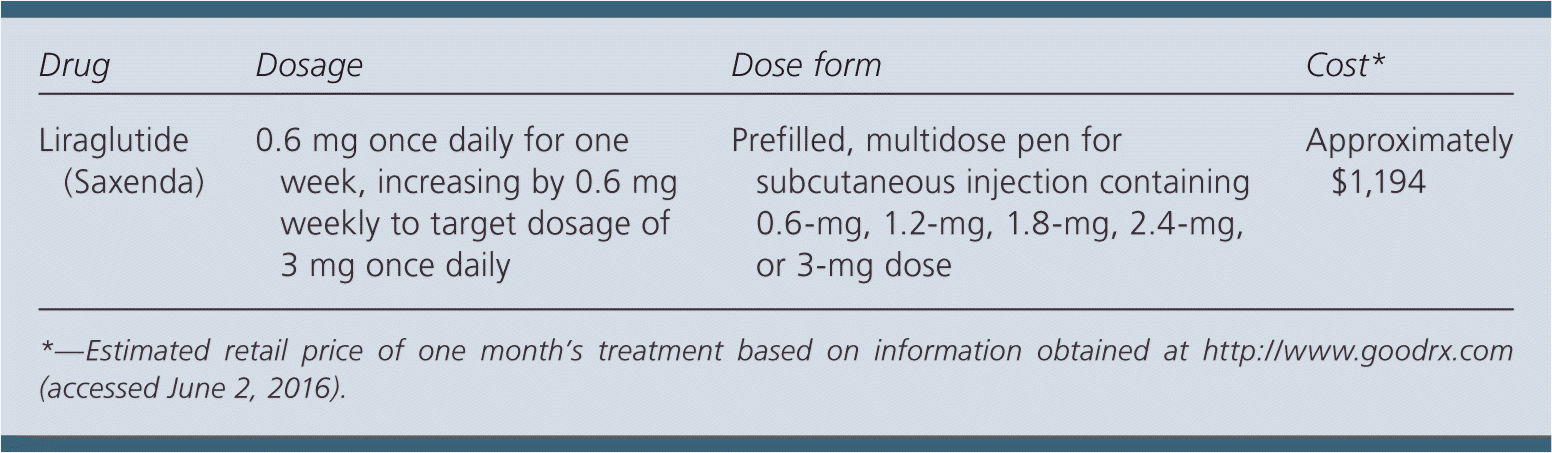
| Drug | Dosage | Dose form | Cost* |
|---|---|---|---|
| Liraglutide (Saxenda) | 0.6 mg once daily for one week, increasing by 0.6 mg weekly to target dosage of 3 mg once daily | Prefilled, multidose pen for subcutaneous injection containing 0.6-mg, 1.2-mg, 1.8-mg, 2.4-mg, or 3-mg dose | Approximately $1,194 |
SAFETY
Safety concerns with liraglutide include acute gallbladder disease, acute pancreatitis, and risk of severe hypoglycemia.1–3 Gallbladder disease and cholecystitis can occur in patients rapidly losing weight by any means, but the rates are higher in patients using liraglutide (number needed to harm [NNH] = 100 and 250, respectively) and rise with increasing weight loss.1,2 The risk of pancreatitis is relatively low (0.3%), but transient elevations of pancreatic enzymes of uncertain clinical significance can occur.1–3 Although sulfonylureas and other insulin secretagogues are not contraindicated for use with liraglutide, their dose should be lowered by at least 50% if they are continued while patients are taking liraglutide. Both severe (NNH = 143) and symptomatic (NNH = 10) hypoglycemia have occurred in patients with type 2 diabetes who are taking sulfonylureas and liraglutide for weight loss.1,2 Liraglutide should not be used in patients taking insulin.1,2 Kidney injury may occur in volume-depleted patients.1 Liraglutide is contraindicated in patients with a family or personal history of medullary thyroid cancers as well as multiple endocrine neoplasia syndrome type 2 because of reports of thyroid tumors in animals.1 As with other GLP-1 receptor agonists, transient pulse elevations of uncertain clinical significance have been reported, with return to baseline after cessation of liraglutide therapy.1–3 Long-term safety (greater than two years) has not been studied.
Liraglutide is a U.S. Food and Drug Administration pregnancy category X drug. Because it is unknown if the medication is excreted in breast milk, it should not be taken by breastfeeding women. Liraglutide is not approved for use in children.
TOLERABILITY
Gastrointestinal symptoms are common, and approximately 10% of patients will stop treatment because of adverse effects.2–4 Nausea (39%), diarrhea or constipation (20%), and vomiting (15%) are reported with liraglutide. About one in four patients with type 2 diabetes will experience symptomatic hypoglycemia at least once over the course of one year.1–4
EFFECTIVENESS
Liraglutide has been evaluated in two double-blind studies of more than 3,000 obese or overweight patients with hyperlipidemia, hypertension, or diabetes. In both studies, adding liraglutide to lifestyle counseling for one year resulted in an average 8.9- to 13.3-lb (4- to 6-kg) greater weight loss.2–4 A clinically significant weight loss of 5% of baseline body weight at one year was also consistently achieved by more than one-half of patients treated with liraglutide (number needed to treat [NNT] = 2 to 3).2,3 A 10% loss (approximately 22 lb [10 kg]) was achieved in 25% to 33% of patients (NNT = 4 to 5).2,3 Sustained weight loss for up to two years has been demonstrated with continued use of liraglutide, but weight gain may occur with discontinuation of the drug.2,5 Liraglutide has been shown to be superior to orlistat (Xenical) for achieving clinically significant weight loss at one and two years of use.5
Liraglutide improves cardiometabolic markers such as blood pressure, waist circumference, body mass index, and A1C, although the clinical relevance of these reductions remains uncertain.2,3 Patients may also report improved physical function, as measured by a validated self-reported measure of obesity-related quality of life.2,3 As with other pharmacologic treatments, liraglutide has not been studied to determine its effect on cardiovascular- or diabetes-related morbidity, or overall mortality. It has not been studied when used in conjunction with other obesity treatments.
PRICE
A one-month supply of liraglutide at the target dosage of 3 mg per day costs approximately $1,194. In comparison, orlistat costs $580 for 120 mg three times per day for 30 days and zonisamide (Zonegran) costs $30 ($400) for 100 mg per day for 30 days.
SIMPLICITY
The starting dosage of liraglutide is 0.6 mg daily, injected subcutaneously at any time of day without regard to meals. The dosage should be increased weekly by 0.6 mg until the target dosage of 3 mg per day is reached. Patients who are unable to tolerate the 3-mg dose should stop treatment, as should patients who do not lose 4% of their baseline body weight after four months. If patients achieve clinically significant weight loss after one year, liraglutide may be continued to maintain weight loss. Syringes should be refrigerated until first use, but then may be refrigerated or kept at room temperature for up to 30 days.1
Bottom Line
Liraglutide, combined with lifestyle counseling, produces a clinically significant and sustained weight loss that continues as long as it is used. Nausea and vomiting, however, are common adverse effects, and about one in 10 patients will discontinue treatment. Liraglutide has not been studied to determine its effect on patient-oriented outcomes such as the development of osteoarthritis, prevention of cardiovascular disease, and reduction of mortality.