A more recent article on chronic asthma treatment is available.
Am Fam Physician. 2016;94(6):454-462
Author disclosure: No relevant financial affiliations.
Chronic asthma is a major health concern for children and adults worldwide. The goal of treatment is to prevent symptoms by reducing airway inflammation and hyperreactivity. Step-up therapy for symptom control involves initiation with low-dose treatment and increasing intensity at subsequent visits if control is not achieved. Step-down therapy starts with a high-dose regimen, reducing intensity as control is achieved. Multiple randomized controlled trials have shown that inhaled corticosteroids are the most effective monotherapy. Other agents may be added to inhaled corticosteroids if optimal symptom control is not initially attained. Long-acting beta2 agonists are the most effective addition, but they are not recommended as monotherapy because of questions regarding their safety. Leukotriene receptor antagonists can be used in addition to inhaled corticosteroids, but they are not as effective as adding a long-acting beta2 agonist. Patients with mild persistent asthma who prefer not to use inhaled corticosteroids may use leukotriene receptor antagonists as monotherapy, but they are less effective. Because of their high cost and a risk of anaphylaxis, monoclonal antibodies should be reserved for patients with severe symptoms not controlled by other agents. Immunotherapy should be considered in persons with asthma triggered by confirmed allergies if they are experiencing adverse effects with medication or have other comorbid allergic conditions. Many patients with asthma use complementary and alternative agents, most of which lack data regarding their safety or effectiveness.
Approximately 25.7 million persons in the United States, including 7 million children, had the diagnosis of asthma as of 2010.1 It is reported that 4.1 million children experienced at least one asthma exacerbation in 2011.2 Between 1995 and 2010, exacerbations accounted for one-third of all hospital admissions for children younger than 15 years.3 Asthma caused 3,345 U.S. deaths in 2011,4 and it accounts for $50.1 billion annually in direct health care costs.5 The management of asthma involves care plans, chronic medications, and monitoring and self-care for acute exacerbations. Therapeutic agents used in the chronic management of asthma aim to prevent symptoms by controlling airway inflammation and hyperreactivity. This article reviews the currently available medications and complementary agents for chronic asthma management. A previous article in American Family Physician discussed the management of acute exacerbations.6
| Clinical recommendation | Evidence rating | References |
|---|---|---|
| Inhaled corticosteroids improve asthma control and quality of life and reduce asthma symptom severity, systemic steroid use, emergency department visits and hospitalizations, and deaths. | A | 10, 15–18 |
| Long-acting beta2 agonists are effective for control of persistent asthma symptoms and are the preferred agents to add to inhaled corticosteroids in patients 12 years and older, but they are not recommended for use as monotherapy. | A | 10, 27, 29 |
| Leukotriene receptor antagonists can be used as adjunctive therapy with inhaled corticosteroids, but they are less effective than long-acting beta2 agonists in patients 12 years and older. | B | 10, 15, 26 |
| If adequate symptom control is not attained with low-dose inhaled corticosteroids, either increasing the inhaled steroid dosage or adding a long-acting beta2 agonist to therapy is appropriate according to current guideline recommendations. | B | 10, 30 |

| Recommendation | Sponsoring organization |
|---|---|
| Do not diagnose or manage asthma without spirometry. | American Academy of Allergy, Asthma and Immunology |
Assessment
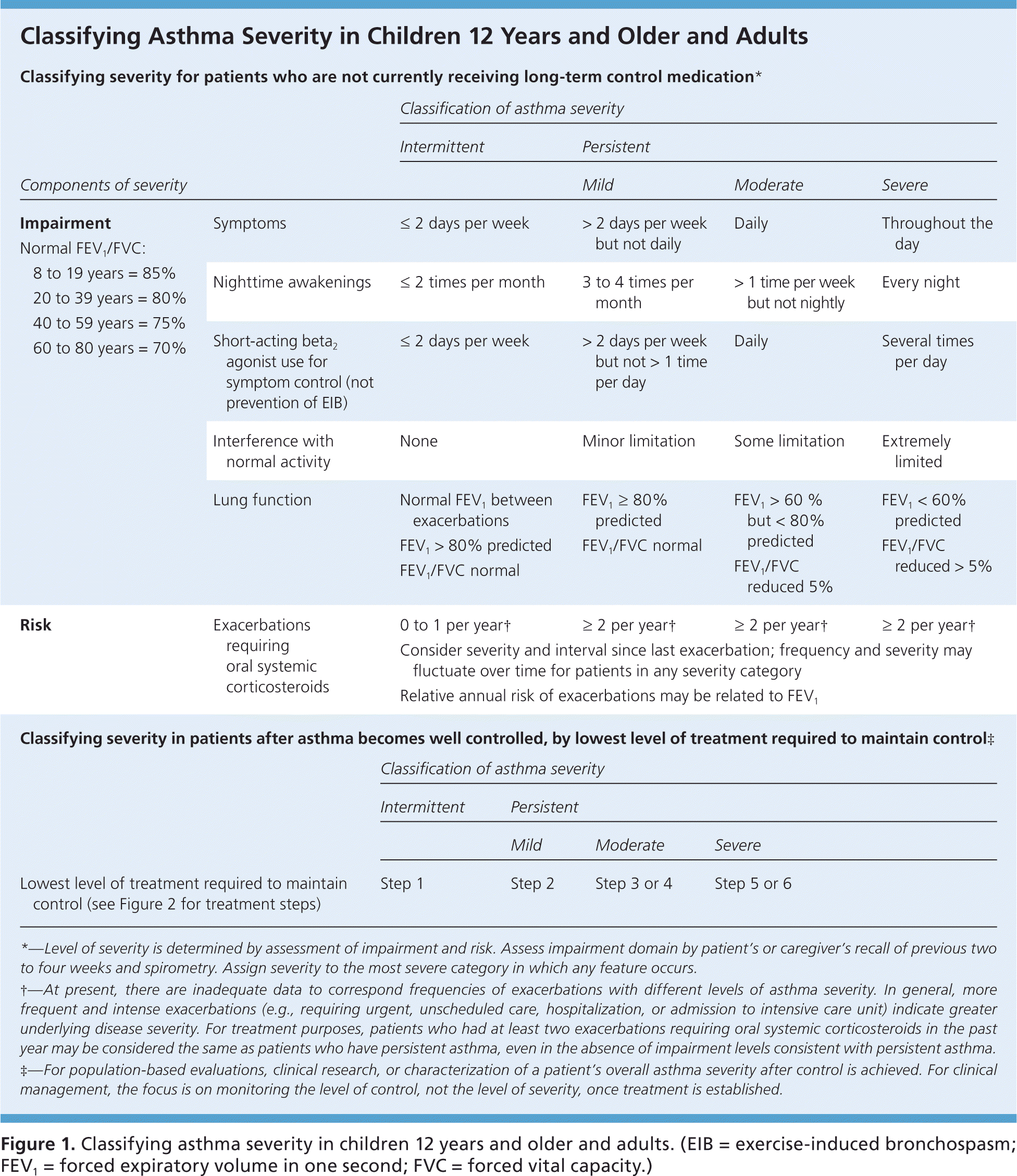
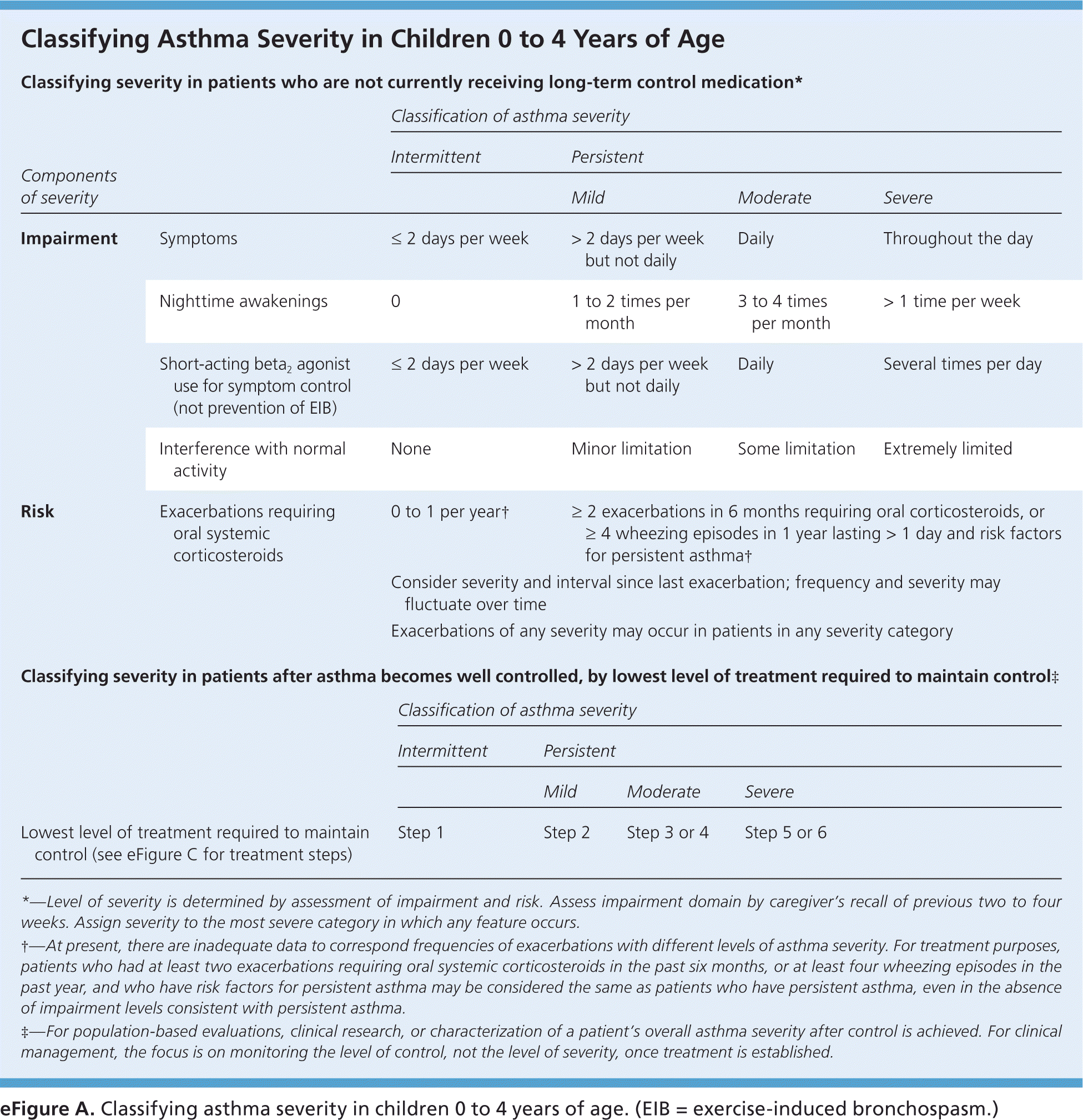
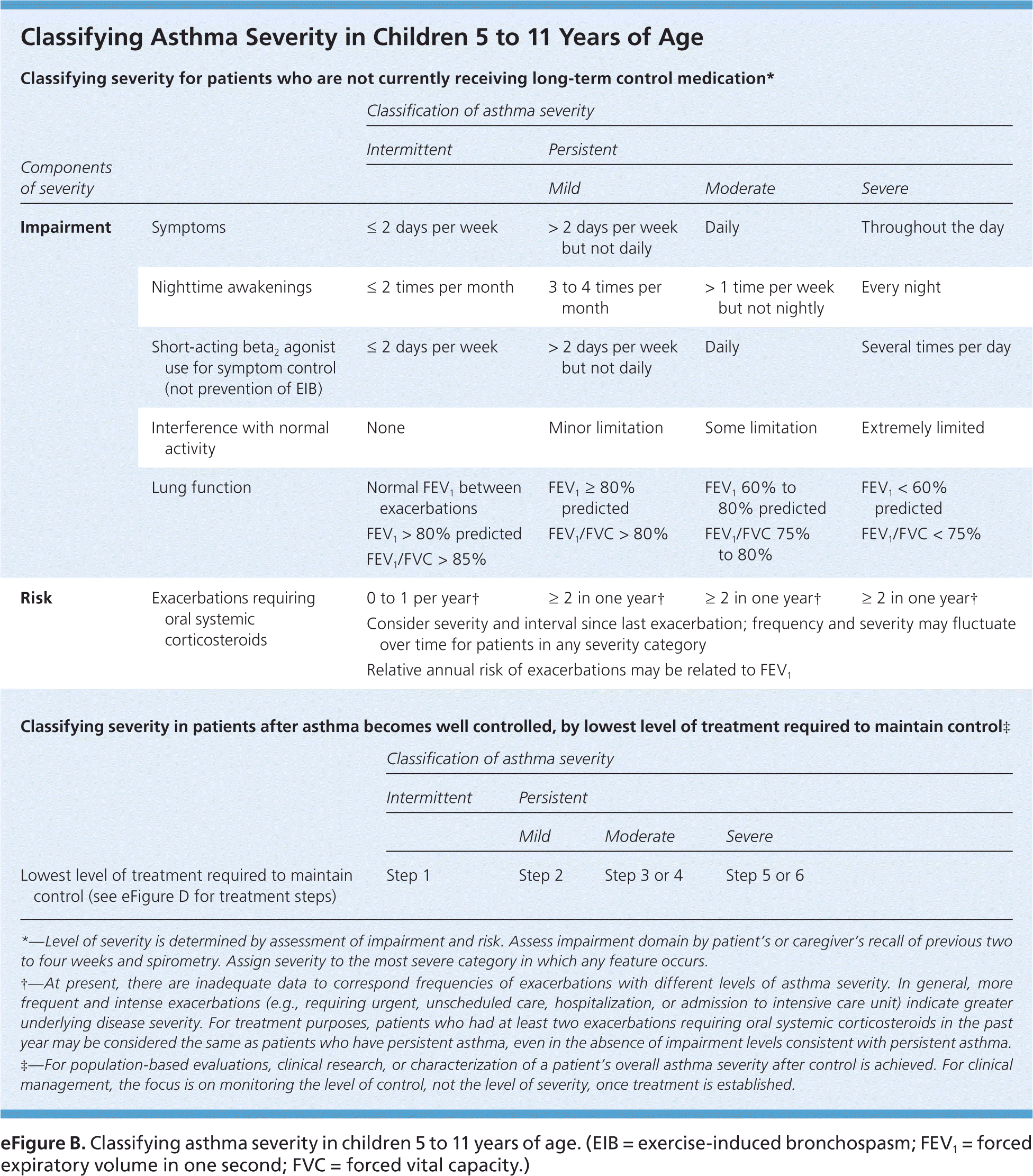
To provide appropriate long-term medication, physicians should assess asthma severity and symptom control at diagnosis and at each subsequent visit using one of several validated tools, such as the Asthma Control Test (https://www.asthma.com/additional-resources/asthma-control-test.html).7–9 The 2007 National Heart, Lung, and Blood Institute/National Asthma Education and Prevention Program Expert Panel Report 3 (EPR-3) recommends classifying disease severity based on level of impairment and risk of adverse events (Figure 1, eFigure A, and eFigure B).10 Once disease severity is determined, the physician must then decide on medication and self-care management options.
Step-Up and Step-Down Therapy
Two general approaches when choosing asthma medication regimens are step-up and step-down therapy (Figure 2, eFigure C, and eFigure D).10 Step-up therapy involves initiating treatment at a low dose and assessing symptom control at subsequent visits (every two to four weeks), increasing the intensity of therapy as needed if control is not initially achieved. Step-down therapy starts with patients receiving a high-dose regimen, the intensity of which is reduced as control is achieved. The latter approach could be preferred, for example, to obtain rapid control in a patient who has significant symptoms at the time of diagnosis. Steps 4 and 5 within the EPR-3 Stepwise Approaches, which recommend the use of a medium- or high-dose inhaled corticosteroid plus a long-acting beta2 agonist (LABA), are common starting points in step-down therapy.
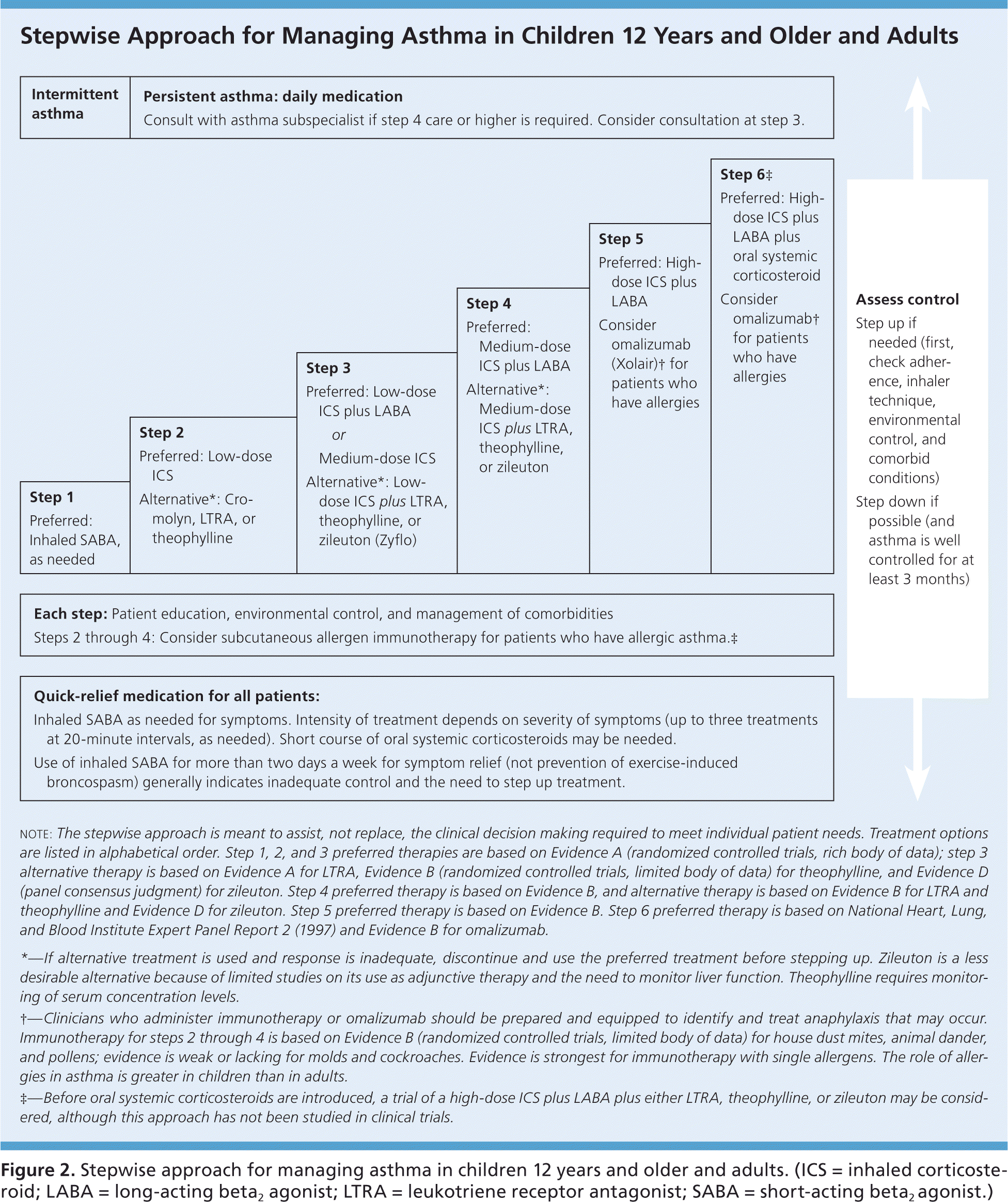
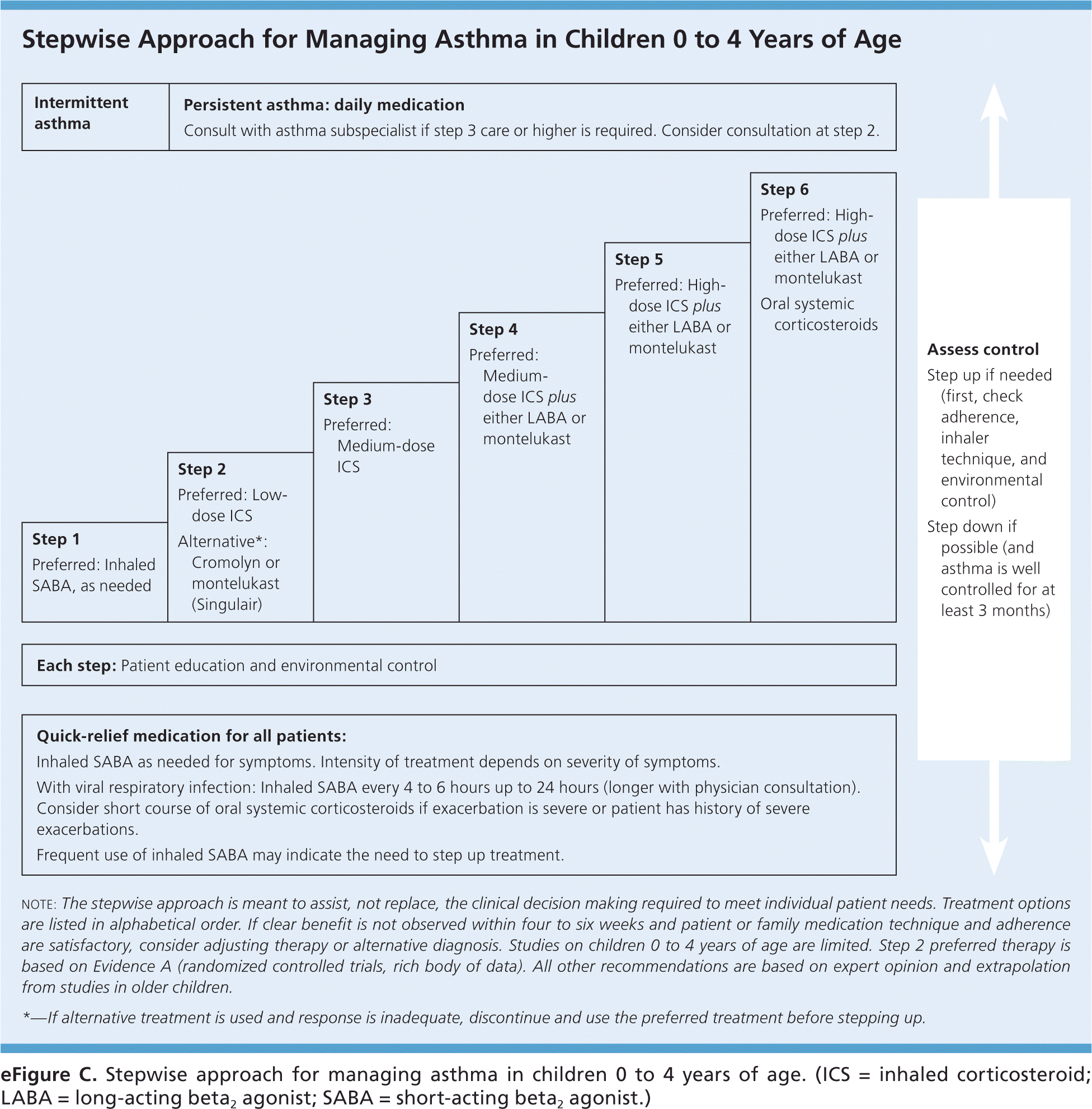
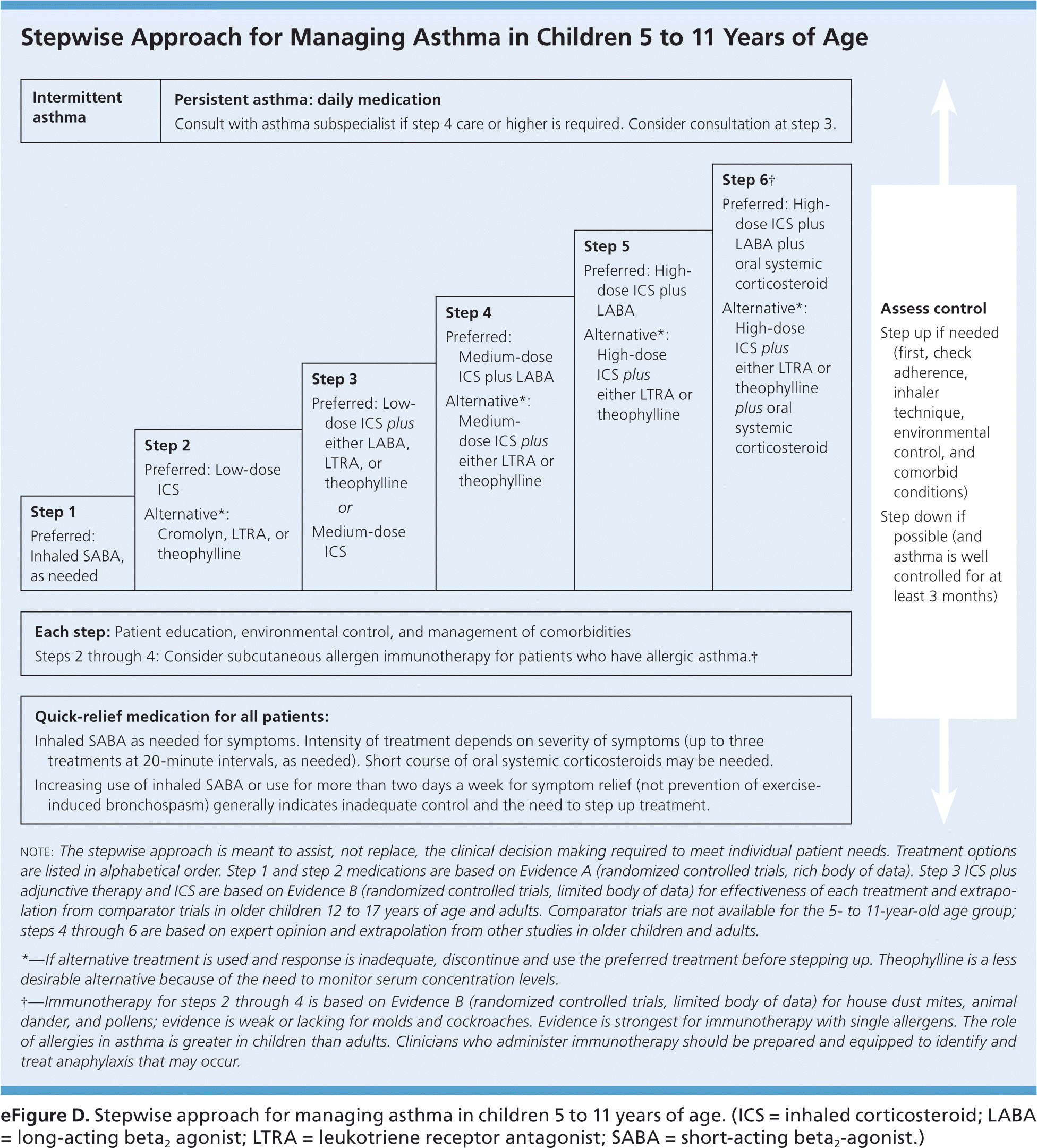
A small randomized trial found that patients with moderate persistent asthma who were started on a high-dose corticosteroid followed by the step-down approach experienced a more prompt improvement in respiratory function and asthma symptoms, as well as a lower maintenance dose of inhaled corticosteroids, compared with patients treated with a step-up approach.11 The EPR-3 guidelines advise that treatment generally be maintained at a high-dose level with patients experiencing good symptom control for three months before stepping down in intensity; reliable patients with well-controlled asthma may be able to step down earlier. Physicians should monitor symptom control in the period after a step down in therapy because patients may have increased symptoms, particularly when an LABA is discontinued.12–14 Medications commonly used in these two approaches are listed in Table 1, with additional information on dosing and adverse effects for these drugs available in eTable A.
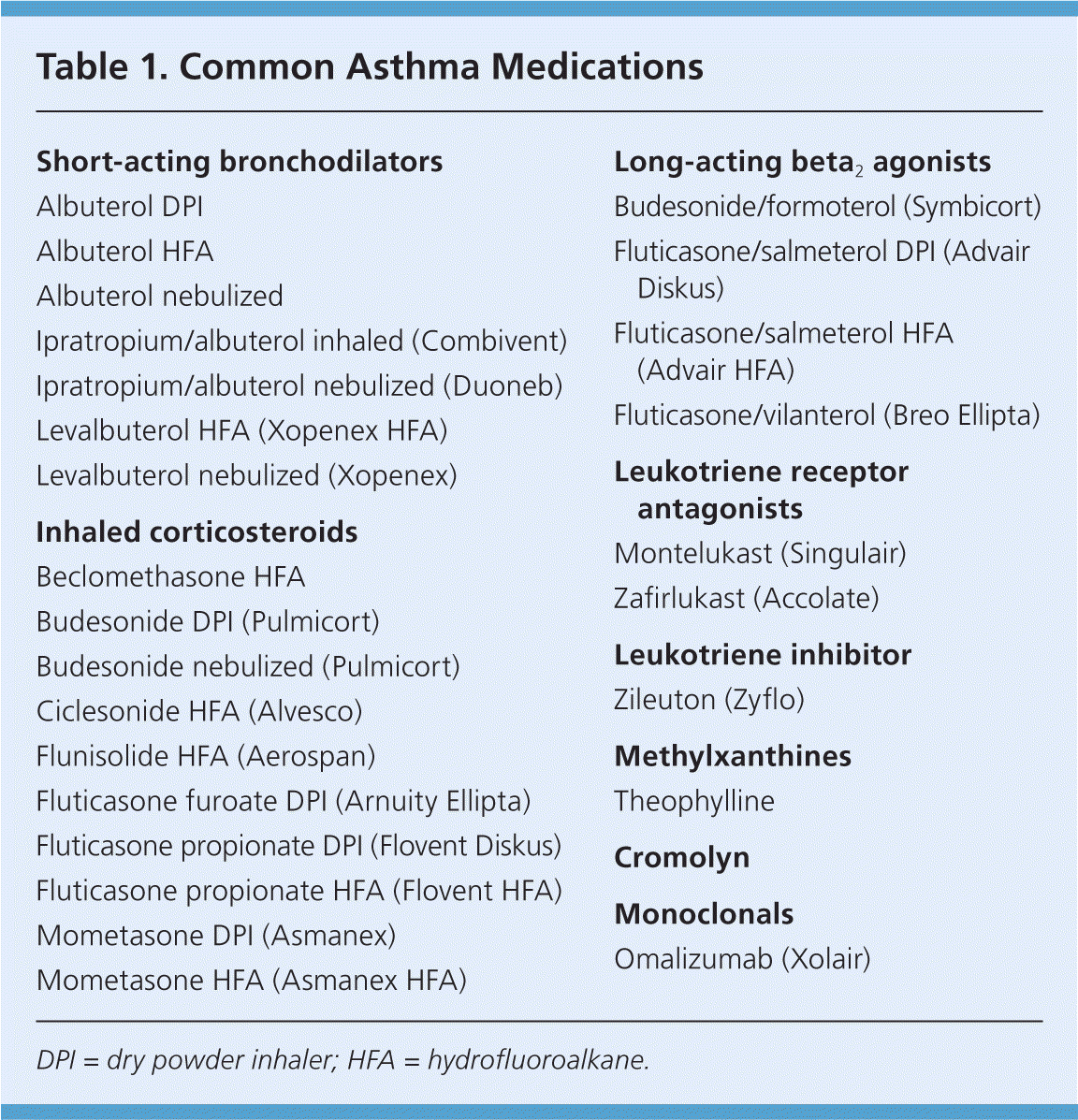
| Short-acting bronchodilators |
| Albuterol DPI |
| Albuterol HFA |
| Albuterol nebulized |
| Ipratropium/albuterol inhaled (Combivent) |
| Ipratropium/albuterol nebulized (Duoneb) |
| Levalbuterol HFA (Xopenex HFA) |
| Levalbuterol nebulized (Xopenex) |
| Inhaled corticosteroids |
| Beclomethasone HFA |
| Budesonide DPI (Pulmicort) |
| Budesonide nebulized (Pulmicort) |
| Ciclesonide HFA (Alvesco) |
| Flunisolide HFA (Aerospan) |
| Fluticasone furoate DPI (Arnuity Ellipta) |
| Fluticasone propionate DPI (Flovent Diskus) |
| Fluticasone propionate HFA (Flovent HFA) |
| Mometasone DPI (Asmanex) |
| Mometasone HFA (Asmanex HFA) |
| Long-acting beta2agonists |
| Budesonide/formoterol (Symbicort) |
| Fluticasone/salmeterol DPI (Advair Diskus) |
| Fluticasone/salmeterol HFA(Advair HFA) |
| Fluticasone/vilanterol (Breo Ellipta) |
| Leukotriene receptor antagonists |
| Montelukast (Singulair) |
| Zafirlukast (Accolate) |
| Leukotriene inhibitor |
| Zileuton (Zyflo) |
| Methylxanthines |
| Theophylline |
| Cromolyn |
| Monoclonals |
| Omalizumab (Xolair) |
| Drug | Dosage | Adverse effects |
|---|---|---|
| Short-acting bronchodilators | ||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Inhaled corticosteroids | ||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Long-acting beta2 agonists | ||
|
|
|
|
|
|
|
|
|
|
|
|
| Leukotriene receptor antagonists | ||
|
|
|
|
|
|
| Leukotriene inhibitor | ||
|
|
|
| Methylxanthines | ||
|
|
|
| Cromolyn |
|
|
| Monoclonals | ||
|
|
|
Inhaled Corticosteroids
Inhaled corticosteroids are the most effective long-term medication for asthma.10,15–18 They have been shown to reduce symptom severity, systemic steroid use, emergency department visits, hospitalizations, and deaths caused by asthma, and improve asthma control, quality of life, and objective measures of lung function.10,15–18 Adverse effects of inhaled corticosteroids are limited, with only a slight effect on linear growth of approximately 0.5 cm per year noted in children. The effect on linear growth lessens after the first year of medication use and seems to be independent of patient age or the type of corticosteroid, dose, or delivery mechanism. It is unclear if inhaled corticosteroid use has an impact on final adult height.19 Other adverse effects, such as dysphonia, are generally self-limited or may be improved by changing the delivery mechanism of the inhaled corticosteroid.20
There are clinically significant differences in patient response to corticosteroids that are associated with age, race, and risk factors such as smoking. Black children and smokers have an increased risk of corticosteroid insensitivity.21,22 In general, delivery mechanism and type of steroid have little impact on the clinical effectiveness of corticosteroids, with the notable exception of a spacer device, which can result in a 20% to 30% increase in the amount of medication that is deposited in the lungs.23 Dosing of inhaled corticosteroids should be managed in a step-up or step-down fashion based on an assessment of symptom control and severity (Figure 2, eFigure C, and eFigure D).10 Whereas abrupt cessation of inhaled corticosteroids predisposes patients to acute asthma exacerbations, changing the dosage of an inhaled corticosteroid does not increase exacerbation risk.24,25
Long-Acting Beta2 Agonists
LABAs are effective for the control of persistent asthma symptoms. They initially have an action of more than 12 to 24 hours. Available non-combination LABAs include salmeterol (Serevent) and formoterol (Foradil). Duration of action decreases to less than five hours with chronic regular use of LABAs,10 excluding those that contain vilanterol which currently lack data regarding duration of action decrease. The addition of an LABA to inhaled corticosteroid therapy is superior to the addition of leukotriene receptor antagonists (LTRAs) to inhaled corticosteroids in reducing asthma exacerbations requiring oral corticosteroid use, as well as improving quality-of-life measures and the effects and frequency of rescue inhaler use.26 Current evidence shows no clear difference in the risk of fatal adverse events between LABA monotherapy and combination therapy with inhaled corticosteroids. The risk of nonfatal adverse events is increased with salmeterol monotherapy, but it is not significantly increased with either formoterol monotherapy or combination therapy with inhaled corticosteroids and either LABA option.27 Current recommendations discourage the use of LABA monotherapy for long-term control of asthma.10
Combination Therapy
The combination of an inhaled corticosteroid and an LABA is considered a preferred therapy by the EPR-3 for the control of moderate persistent asthma in children five to 11 years of age and those 12 years and older.10 Combination therapy offers the best prevention of severe asthma exacerbations.28 A 2013 study confirmed the overall safety of combination inhaled corticosteroid and LABA therapy, especially compared with LABA monotherapy.29 Combination therapy dosing should be managed in a step-up or step-down approach similar to the management of inhaled corticosteroid therapy. Slight differences in when to start combination therapy are noted between the EPR-3 and Global Initiative for Asthma (GINA) guidelines.10,30 For example, according to step 3 of the EPR-3 stepwise approach for patients 12 years and older, either a low-dose inhaled corticosteroid plus an LABA, or a medium-dose inhaled corticosteroid alone is appropriate (Figure 2).10 The GINA guidelines recommend a low-dose inhaled corticosteroid plus an LABA as the preferred selection in this age group, with a medium-dose inhaled corticosteroid considered the secondary option.
Leukotriene Modifiers
Leukotriene modifiers include LTRAs and leukotriene inhibitors, which both act as anti-inflammatory medications. LTRAs block leukotriene receptors, whereas leukotriene inhibitors block the production of 5-lipoxygenase. The two LTRAs licensed in the United States are montelukast (Singulair) and zafirlukast (Accolate). LTRAs may be used as monotherapy for mild persistent asthma, but are considered second-line agents based on the EPR-310 and GINA guidelines.30 For mild to moderate asthma, the risk of exacerbation is approximately 50% less in patients prescribed an inhaled corticosteroid compared with those prescribed an LTRA.15 A 2014 Cochrane review found an LABA plus inhaled corticosteroid to be modestly superior to an LTRA plus inhaled corticosteroid in adults with inadequately controlled asthma.26 LTRAs are best used to improve pulmonary function in patients with aspirin-sensitive asthma31 and to decrease symptoms in exercise-induced bronchospasm.32,33 They should also be considered in patients with mild persistent asthma who prefer not to use inhaled corticosteroids. Although LTRAs generally have few adverse effects, physicians should be aware of rare case reports of eosinophilic granulomatosis with polyangiitis (Churg-Strauss syndrome), psychiatric symptoms, hypertriglyceridemia, angioedema, urticaria, and glomerulonephritis.34
Leukotriene inhibitors, such as zileuton (Zyflo), are a more recent addition to the treatment of asthma. Limited data show some improvement in peak flows with zileuton compared with montelukast.35 However, zileuton is extremely expensive and has not been shown to improve symptom scores.
Methylxanthines
Theophylline, the most commonly used methylxanthine in asthma patients, acts as a bronchodilator at high serum concentrations (10 to 20 mcg per L [56 to 111 μmol per L]), but has an anti-inflammatory effect at lower serum concentrations (5 to 10 mcg per L [28 to 56 μmol per L]).36,37 Theophylline administered with inhaled corticosteroids decreases exacerbations,38 but it has similar effects to increasing the dosage of the inhaled corticosteroid.39,40 The EPR-3 specifies that theophylline is a nonpreferred alternative to inhaled corticosteroid.10 The GINA guidelines recommend a trial of increased dosage of inhaled corticosteroid before considering theophylline, unless steroid sparing is necessary, such as in patients with severe glaucoma or active tuberculosis infection.30 Patients in developing countries are more likely to use low-dose theophylline than inhaled corticosteroids because it is a cheaper option.39,40 Although theophylline is considered safer at lower serum concentrations, care of patients who use theophylline should be comanaged with an asthma subspecialist because of the narrow therapeutic range of this drug and the risk of death from an overdose.36,40 Theophylline is metabolized in the liver and is susceptible to drug-drug interactions through cytochrome P450 1A2 (Table 2).37,41
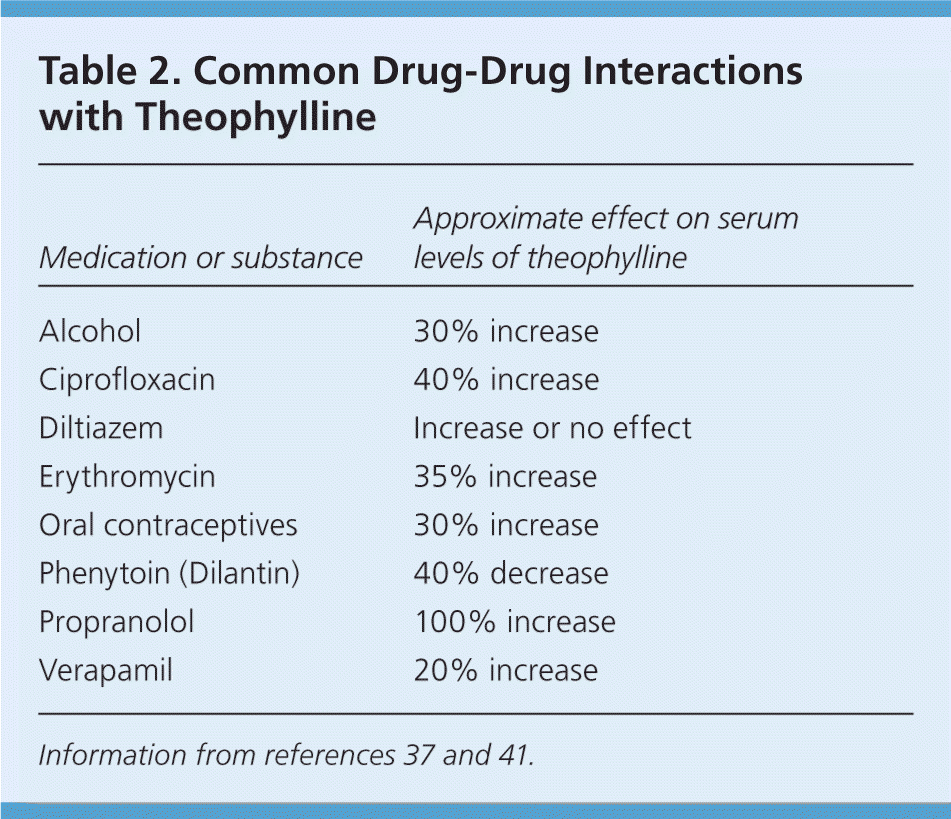
| Medication or substance | Approximate effect on serum levels of theophylline |
|---|---|
| Alcohol | 30% increase |
| Ciprofloxacin | 40% increase |
| Diltiazem | Increase or no effect |
| Erythromycin | 35% increase |
| Oral contraceptives | 30% increase |
| Phenytoin (Dilantin) | 40% decrease |
| Propranolol | 100% increase |
| Verapamil | 20% increase |
Cromolyn
Cromolyn decreases bronchospasm through an anti-inflammatory effect.42 A 2008 Cochrane review found insufficient evidence of benefit of cromolyn over placebo.43 Because cromolyn is less effective and less cost-effective than an inhaled corticosteroid, its use should be limited to patients who cannot tolerate inhaled corticosteroids.44 Cromolyn is beneficial for exercise-induced bronchospasm but is considered second-line therapy.44,45
Monoclonal Antibodies
Omalizumab (Xolair) is currently the only monoclonal anti-immunoglobulin E (IgE) antibody with a U.S. Food and Drug Administration indication for asthma.46 It binds the free IgE antibodies, decreasing the release of inflammatory mediators from mast cells. In a randomized trial, omalizumab reduced the rate of exacerbations in inner-city children from 48.8% to 30.3%, resulting in decreased reliance on an inhaled corticosteroid.47 A 2014 Cochrane review found omalizumab effective in reducing exacerbations, decreasing the dosage of inhaled corticosteroid used, and improving health-related quality of life.48 Because of its high cost and the risk of anaphylaxis, omalizumab should be considered only for adults and children 12 years and older with confirmed IgE-dependent allergic asthma that is uncontrolled with conventional medications.49,50
Immunotherapy
Subcutaneous and sublingual immunotherapies involve repeated patient exposure to antigens to desensitize the patient to the antigen. Immunotherapy is effective in reducing exacerbations, need for medication use, and overall cost of care in patients with allergic asthma.51–53 A 2010 Cochrane review found a number needed to treat of 4 to avoid one deterioration in asthma symptoms, but it could not determine the size of effect compared with other therapies.54 Immunotherapy should be considered in patients with asthma triggered by confirmed allergies who are experiencing adverse effects from medication or have other comorbid allergic conditions.
Alternative Treatments
The rate of complementary and alternative medicine (CAM) use in children and adolescents with asthma is as high as 71% to 84%, but 54% of parents do not disclose the use of these methods.55,56 CAM use is more common among children with poorly controlled asthma and those with barriers to treatment.57,58 However, data indicate that CAM treatment is typically not used as a substitute for conventional medicine.57 Patients who are receiving CAM substances should be cautioned that there is little regulation to ensure the consistency and purity of the contents and that CAM is never a substitute for rescue medication. Common CAM treatments and their effects on asthma symptoms are listed in Table 3.59–74
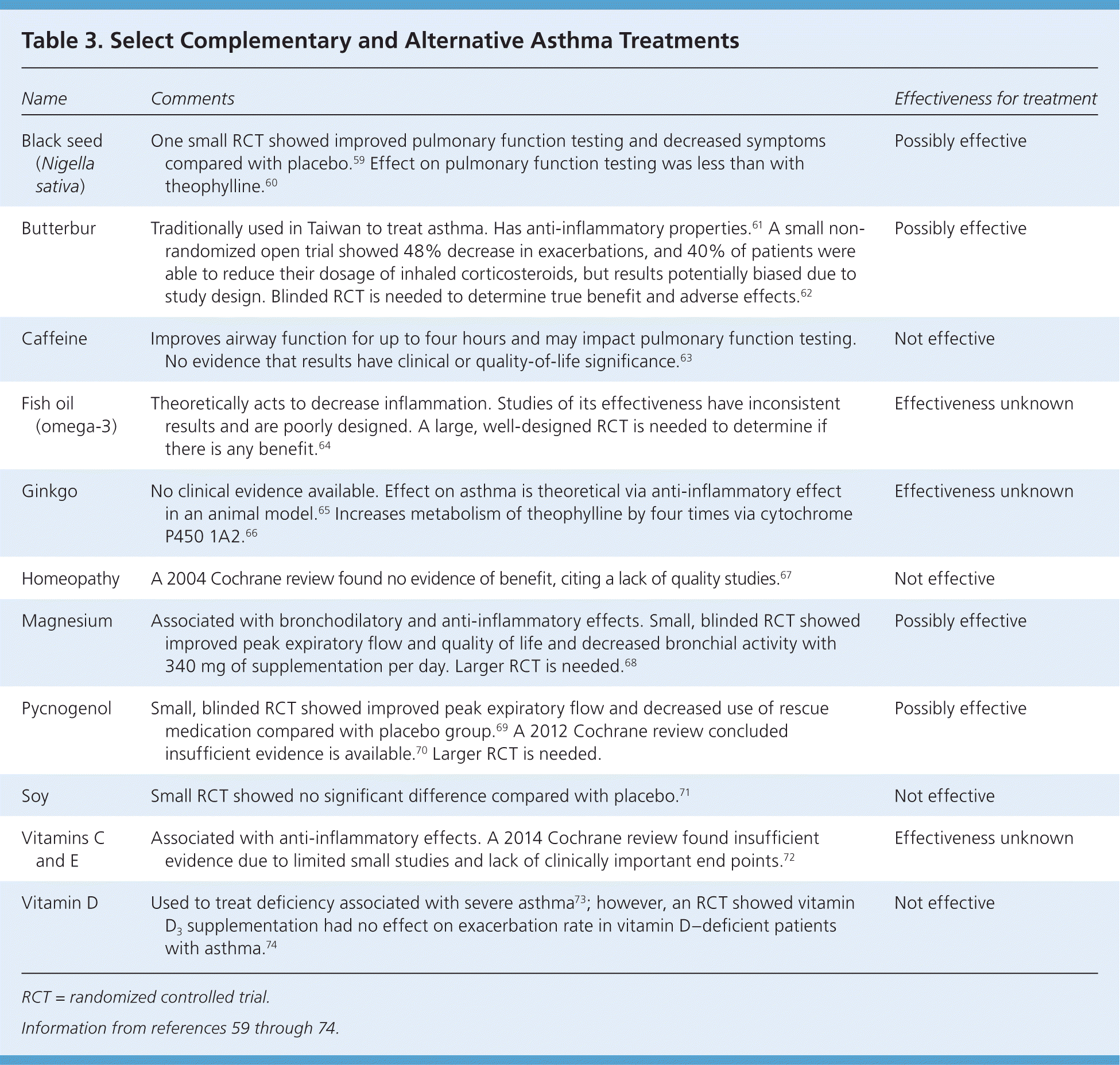
| Name | Comments | Effectiveness for treatment |
|---|---|---|
| Black seed (Nigella sativa) | One small RCT showed improved pulmonary function testing and decreased symptoms compared with placebo.59 Effect on pulmonary function testing was less than with theophylline.60 | Possibly effective |
| Butterbur | Traditionally used in Taiwan to treat asthma. Has anti-inflammatory properties.61 A small non-randomized open trial showed 48% decrease in exacerbations, and 40% of patients were able to reduce their dosage of inhaled corticosteroids, but results potentially biased due to study design. Blinded RCT is needed to determine true benefit and adverse effects.62 | Possibly effective |
| Caffeine | Improves airway function for up to four hours and may impact pulmonary function testing. No evidence that results have clinical or quality-of-life significance.63 | Not effective |
| Fish oil (omega-3) | Theoretically acts to decrease inflammation. Studies of its effectiveness have inconsistent results and are poorly designed. A large, well-designed RCT is needed to determine if there is any benefit.64 | Effectiveness unknown |
| Ginkgo | No clinical evidence available. Effect on asthma is theoretical via anti-inflammatory effect in an animal model.65 Increases metabolism of theophylline by four times via cytochrome P450 1A2.66 | Effectiveness unknown |
| Homeopathy | A 2004 Cochrane review found no evidence of benefit, citing a lack of quality studies.67 | Not effective |
| Magnesium | Associated with bronchodilatory and anti-inflammatory effects. Small, blinded RCT showed improved peak expiratory flow and quality of life and decreased bronchial activity with 340 mg of supplementation per day. Larger RCT is needed.68 | Possibly effective |
| Pycnogenol | Small, blinded RCT showed improved peak expiratory flow and decreased use of rescue medication compared with placebo group.69 A 2012 Cochrane review concluded insufficient evidence is available.70 Larger RCT is needed. | Possibly effective |
| Soy | Small RCT showed no significant difference compared with placebo.71 | Not effective |
| Vitamins C and E | Associated with anti-inflammatory effects. A 2014 Cochrane review found insufficient evidence due to limited small studies and lack of clinically important end points.72 | Effectiveness unknown |
| Vitamin D | Used to treat deficiency associated with severe asthma73; however, an RCT showed vitamin D3 supplementation had no effect on exacerbation rate in vitamin D–deficient patients with asthma.74 | Not effective |
Data Sources: A PubMed search was completed in Clinical Queries using the key terms asthma, inhaled corticosteroids, leukotriene receptor antagonist, long-acting beta2 agonists, and omalizumab. The search included meta-analyses, randomized controlled trials, clinical trials, and reviews. Also searched were Cochrane Database of Systematic Reviews, Essential Evidence Plus, and Natural Medicines Comprehensive Database. Search dates: January 15, 2015 and August 20, 2015.
The opinions and assertions contained herein are the private views of the authors and are not to be construed as official or as reflecting the views of the U.S. Air Force Medical Department or the U.S. Air Force at large.