Am Fam Physician. 2016;94(6):512-513
Author disclosure: No relevant financial affiliations.
Ivermectin 1% cream (Soolantra) is a topical prescription medication labeled for the treatment of inflammatory lesions of rosacea. Its mechanism of action is unknown1 but may be due to a combination of its anti-inflammatory effects and its antiparasitic effects on the Demodex mite, which lives on the skin and may contribute to the symptoms of rosacea.2
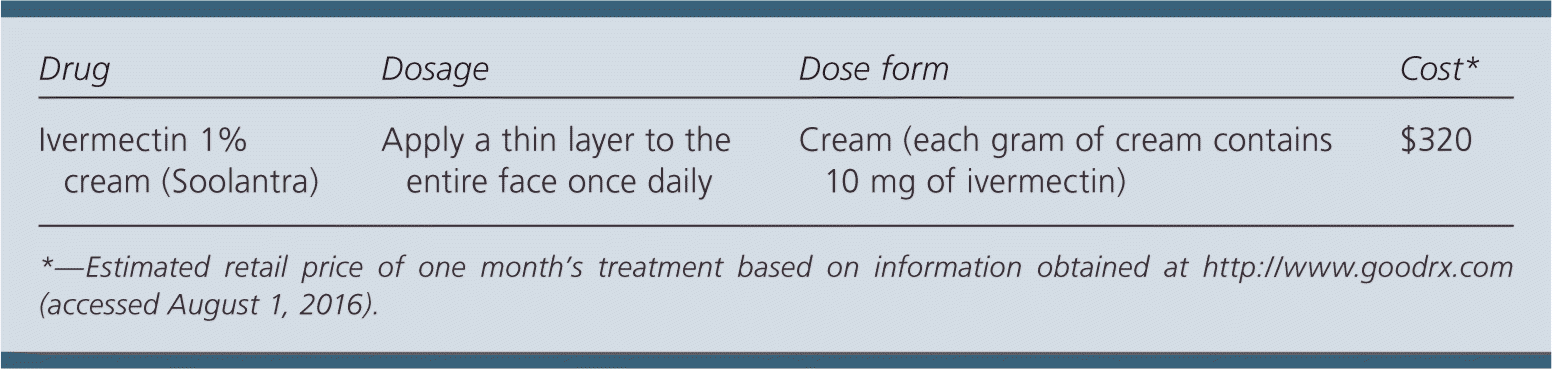
| Drug | Dosage | Dose form | Cost* |
|---|---|---|---|
| Ivermectin 1% cream (Soolantra) | Apply a thin layer to the entire face once daily | Cream (each gram of cream contains 10 mg of ivermectin) | $320 |
SAFETY
Studies have shown ivermectin cream to be safe with no serious adverse effects. In randomized trials, the rate of adverse effects was similar to those of vehicle, metronidazole gel, and azelaic acid (Azelex), and no systemic adverse effects occurred.3–5 Two trials that studied 707 patients for up to one year revealed no safety concerns.5 Ivermectin cream is a U.S. Food and Drug Administration pregnancy category C drug.
TOLERABILITY
Ivermectin cream is generally well tolerated. About one in 77 patients will stop using ivermectin cream by 16 weeks because of adverse effects.3,4 For patients using ivermectin cream for up to one year, one in 83 will discontinue treatment because of adverse effects.5 Pooled drop-out rates are similar for patients using vehicle, metronidazole cream, or azelaic acid.3–5 A small proportion of patients (less than 2%) will experience local adverse effects such as a burning skin sensation (1.3%), skin irritation (1%), pruritus (0.8%), and dry skin (0.7%).6 These effects are usually transient and will decrease over time.
EFFECTIVENESS
Ivermectin cream will produce clearing or almost clearing of rosacea lesions in 40% to 80% of patients with moderate to severe symptoms after three months of treatment (number needed to treat [NNT] = 4 to 5).3,4 These results were demonstrated in two trials comparing ivermectin cream with placebo cream in 1,371 patients with moderate to severe papulopustular rosacea. Patients using ivermectin cream most often reported their symptom improvement as “good” or “excellent” (NNT = 3). Patients reporting that rosacea had negatively affected their quality of life (NNT = 5) was less common.4 Ivermectin was also reported to be more effective than twice-daily topical metronidazole (NNT = 11) after four months of treatment in patients with moderate to severe papulopustular rosacea.3 Following discontinuation of treatment, symptoms will return within four months in about one-half of patients.7 Ivermectin cream has not been studied in patients with milder forms of rosacea.
PRICE
A one-month supply of ivermectin cream (one 30-g tube) is about $320. In comparison, other topical treatments cost approximately $120 for one 45-g tube of metronidazole cream and $360 for one 30-g tube of azelaic acid. Oral doxycycline monohydrate is a less expensive off-label option costing about $35 for a one-month supply (100 mg once daily).
SIMPLICITY
Ivermectin cream should be applied once daily in a thin layer covering the entire face. Patients should be instructed to avoid the eyes, lips, and mucosa, and to wash their hands after application.
Bottom Line
Ivermectin cream is an effective treatment for moderate to severe pustular rosacea, but it is more expensive than some other available treatments and has not been studied in patients with milder forms of rosacea. Generic metronidazole cream is a less expensive alternative, and long-term oral doxycycline may be suitable for patients who want a more affordable alternative to a topical agent.