
Am Fam Physician. 2016;94(10):843-846
Author disclosure: No relevant financial affiliations.
Evolocumab (Repatha) is an injectable human monoclonal antibody labeled for decreasing low-density lipoprotein (LDL) cholesterol. Evolocumab is the second approved inhibitor of proprotein convertase subtilisin/kexin type 9 (PCSK9), which prevents the PCSK9 protein from degrading LDL receptors, thereby increasing LDL clearance and reducing serum cholesterol.1 It is labeled for use in combination with a healthy diet and other lipid-lowering therapies in one of three disease conditions: clinical atherosclerotic coronary or vascular disease, heterozygous familial hypercholesterolemia, and homozygous familial hypercholesterolemia.2 As with alirocumab (Praluent), the other injectable monoclonal antibody in this class, evolocumab is not labeled for the prevention of premature mortality or to decrease cardiovascular events.
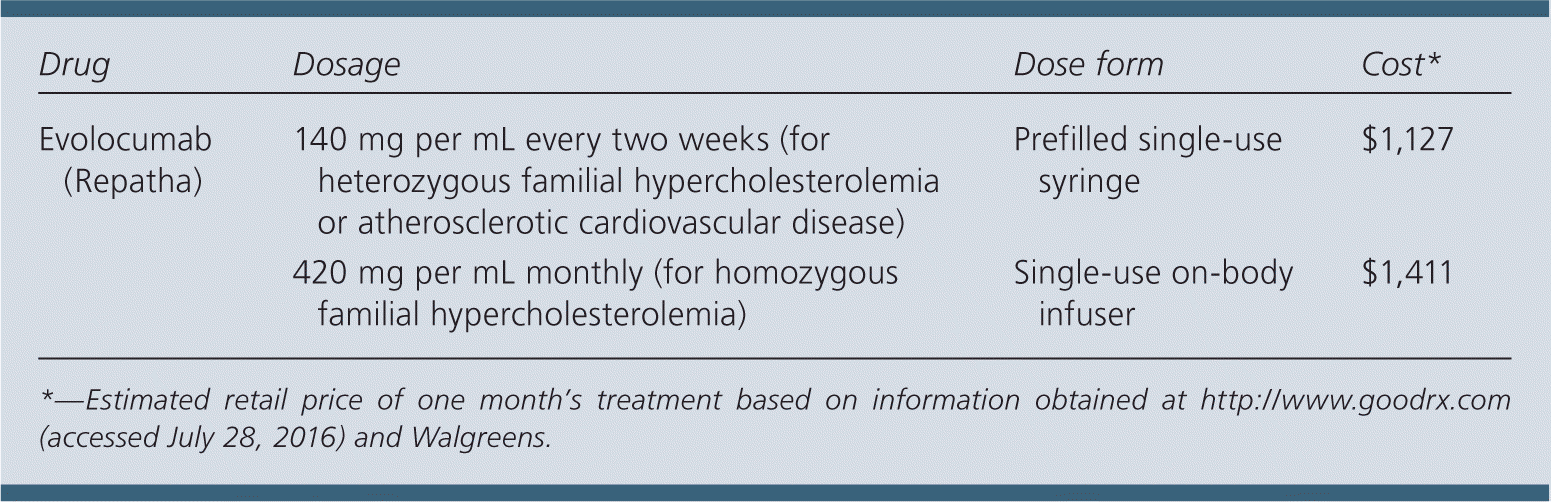
| Drug | Dosage | Dose form | Cost* |
|---|---|---|---|
| Evolocumab (Repatha) | 140 mg per mL every two weeks (for heterozygous familial hypercholesterolemia or atherosclerotic cardiovascular disease) | Prefilled single-use syringe | $1,127 |
| 420 mg per mL monthly (for homozygous familial hypercholesterolemia) | Single-use on-body infuser | $1,411 |
SAFETY
Limited investigation of evolocumab has not identified significant safety issues, and it is unknown if there are any long-term clinically important effects of pharmacologically induced extreme reduction of LDL cholesterol. Evolocumab has not been studied in children younger than 13 years. Because it is a monoclonal antibody, evolocumab has the potential for immunogenicity-related adverse effects. There are no safety data available on women who are pregnant or breastfeeding.
TOLERABILITY
Patients have noted hypersensitivity reactions such as rash (1% vs. 0.5% with placebo) and urticaria (0.4% vs. 0.1% with placebo). Other reported adverse effects include nasopharyngitis (4%), upper respiratory tract infection (2%), and myalgia (1%), at rates similar to those of placebo. About 2% of patients taking evolocumab discontinue the medication because of adverse effects compared with 1% of patients taking placebo.3 The 2% discontinuation rate is similar to that of lipid-lowering medication combinations, such as a statin plus ezetimibe (Zetia).4
EFFECTIVENESS
In patients with atherosclerotic disease or familial hypercholesterolemia who are already taking standard lipid-lowering agents such as statin medications, evolocumab significantly reduces serum LDL cholesterol values by 50% to 75% (from 89 to 124 mg per dL [2.31 to 3.21 mmol per L] to 35 to 49 mg per dL [0.91 to 1.27 mmol per L]) when compared with standard therapy alone at three months to one year.4–6 Although evolocumab is highly effective at numeric reduction of LDL cholesterol, most trials have not investigated clinically important patient-centered outcomes such as myocardial infarction, stroke, or premature death from cardiovascular disease. Although evolocumab is not labeled for the prevention or treatment of cardiovascular events, a single study that preliminarily explored composite cardiovascular outcomes showed that there may be fewer cardiovascular events with evolocumab compared with lipid-lowering therapy. To prevent one additional cardiovascular event, 81 patients must use evolocumab for one year (number needed to treat = 81; Kaplan-Meier estimates are 0.95% for study drug and 2.18% for standard therapy, for composite outcomes).6 It is unknown whether these effects persist beyond one year.
PRICE
A one-month supply of evolocumab costs $1,127 for one injection every two weeks or $1,411 for a single-use on-body infuser. The cost of evolocumab is in addition to other cholesterol-reducing treatments and is similar to that of alirocumab ($1,164 for a one-month supply).
SIMPLICITY
Evolocumab is available in a prefilled single-use syringe. It should be injected once every two weeks (140 mg per mL per dose) when treating heterozygous familial hypercholesterolemia or atherosclerotic cardiovascular disease. When treating homozygous familial hypercholesterolemia, evolocumab may be administered once every month using a single-use on-body infuser (total dose of 420 mg).
Bottom Line
Evolocumab is generally safe and effective at lowering serum LDL cholesterol levels for select patients who need marked cholesterol reduction and can tolerate injections. However, it remains unknown whether evolocumab prevents premature death from cardiovascular events. The role of evolocumab may be limited to use by subspecialists seeing patients with specific conditions requiring dramatic reduction in LDL cholesterol levels.
