Am Fam Physician. 2017;95(4):online
As published by the U.S. Preventive Services Task Force.
Summary of Recommendations and Evidence
The USPSTF recommends screening for colorectal cancer starting at age 50 years and continuing until age 75 years (Table 1). A recommendation.
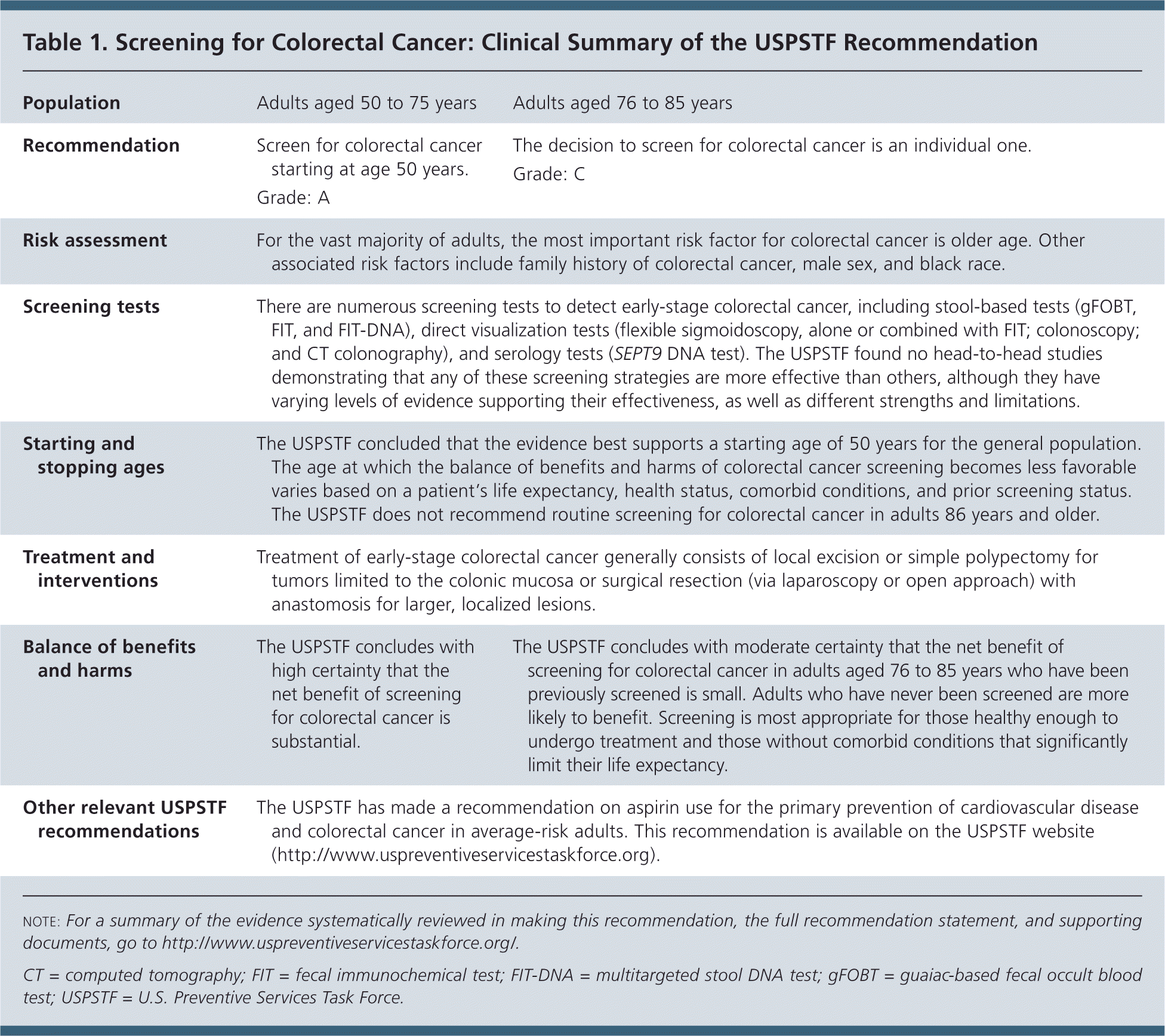
| Population | Adults aged 50 to 75 years | Adults aged 76 to 85 years |
| Recommendation | Screen for colorectal cancer starting at age 50 years. | The decision to screen for colorectal cancer is an individual one. |
| Grade: A | Grade: C | |
| Risk assessment | For the vast majority of adults, the most important risk factor for colorectal cancer is older age. Other associated risk factors include family history of colorectal cancer, male sex, and black race. | |
| Screening tests | There are numerous screening tests to detect early-stage colorectal cancer, including stool-based tests (gFOBT, FIT, and FIT-DNA), direct visualization tests (flexible sigmoidoscopy, alone or combined with FIT; colonoscopy; and CT colonography), and serology tests (SEPT9 DNA test). The USPSTF found no head-to-head studies demonstrating that any of these screening strategies are more effective than others, although they have varying levels of evidence supporting their effectiveness, as well as different strengths and limitations. | |
| Starting and stopping ages | The USPSTF concluded that the evidence best supports a starting age of 50 years for the general population. The age at which the balance of benefits and harms of colorectal cancer screening becomes less favorable varies based on a patient's life expectancy, health status, comorbid conditions, and prior screening status. The USPSTF does not recommend routine screening for colorectal cancer in adults 86 years and older. | |
| Treatment and interventions | Treatment of early-stage colorectal cancer generally consists of local excision or simple polypectomy for tumors limited to the colonic mucosa or surgical resection (via laparoscopy or open approach) with anastomosis for larger, localized lesions. | |
| Balance of benefits and harms | The USPSTF concludes with high certainty that the net benefit of screening for colorectal cancer is substantial. | The USPSTF concludes with moderate certainty that the net benefit of screening for colorectal cancer in adults aged 76 to 85 years who have been previously screened is small. Adults who have never been screened are more likely to benefit. Screening is most appropriate for those healthy enough to undergo treatment and those without comorbid conditions that significantly limit their life expectancy. |
| Other relevant USPSTF recommendations | The USPSTF has made a recommendation on aspirin use for the primary prevention of cardiovascular disease and colorectal cancer in average-risk adults. This recommendation is available on the USPSTF website (http://www.uspreventiveservicestaskforce.org). | |
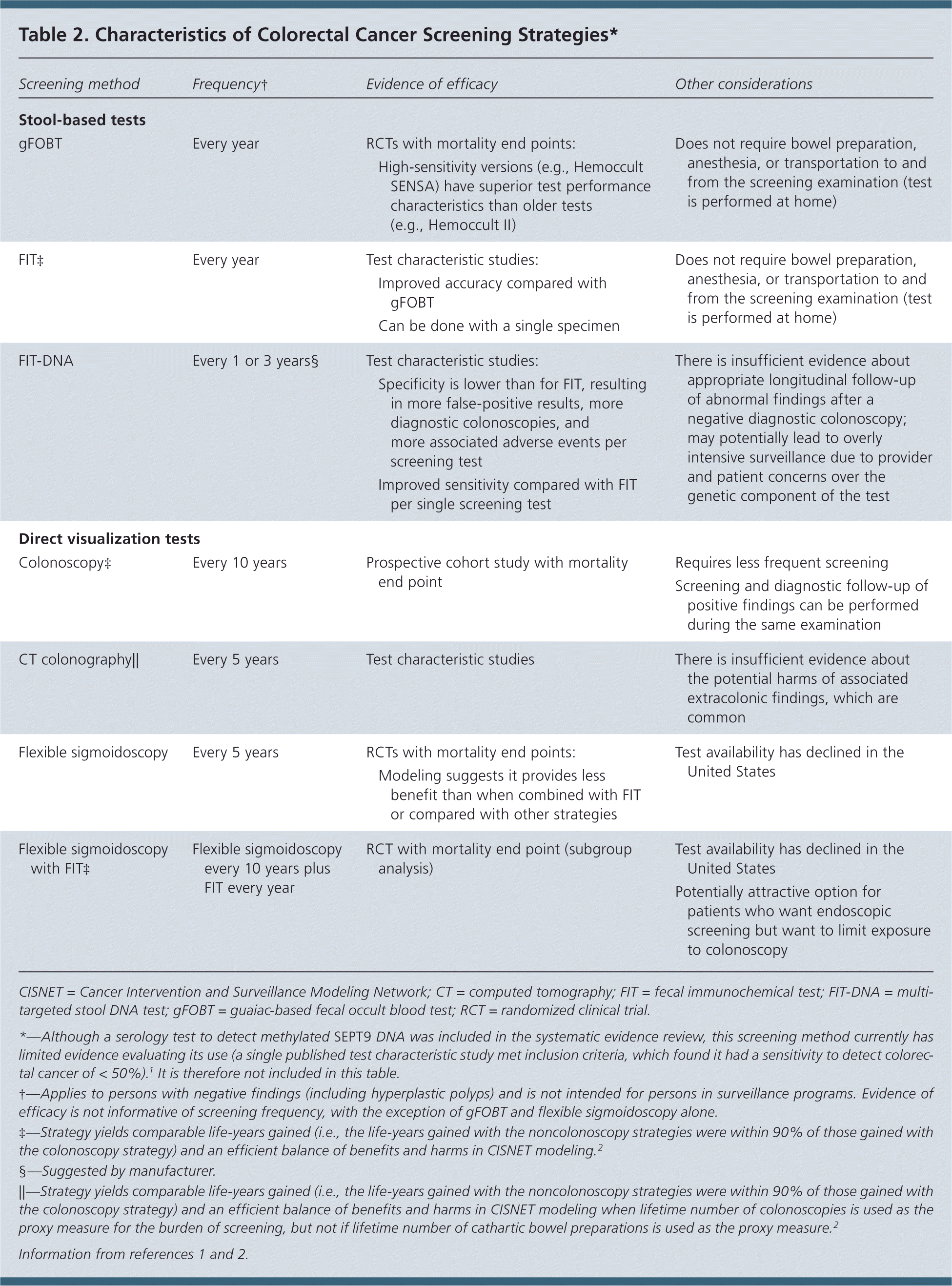
| Screening method | Frequency† | Evidence of efficacy | Other considerations |
|---|---|---|---|
| Stool-based tests | |||
| gFOBT | Every year | RCTs with mortality end points:
| Does not require bowel preparation, anesthesia, or transportation to and from the screening examination (test is performed at home) |
| FIT‡ | Every year | Test characteristic studies:
| Does not require bowel preparation, anesthesia, or transportation to and from the screening examination (test is performed at home) |
| FIT-DNA | Every 1 or 3 years§ | Test characteristic studies:
| There is insufficient evidence about appropriate longitudinal follow-up of abnormal findings after a negative diagnostic colonoscopy; may potentially lead to overly intensive surveillance due to provider and patient concerns over the genetic component of the test |
| Direct visualization tests | |||
| Colonoscopy‡ | Every 10 years | Prospective cohort study with mortality end point | Requires less frequent screening |
| Screening and diagnostic follow-up of positive findings can be performed during the same examination | |||
| CT colonography|| | Every 5 years | Test characteristic studies | There is insufficient evidence about the potential harms of associated extracolonic findings, which are common |
| Flexible sigmoidoscopy | Every 5 years | RCTs with mortality end points: | Test availability has declined in the United States |
| Modeling suggests it provides less benefit than when combined with FIT or compared with other strategies | |||
| Flexible sigmoidoscopy with FIT‡ | Flexible sigmoidoscopy every 10 years plus FIT every year | RCT with mortality end point (subgroup analysis) | Test availability has declined in the United States |
| Potentially attractive option for patients who want endoscopic screening but want to limit exposure to colonoscopy | |||
The decision to screen for colorectal cancer in adults aged 76 to 85 years should be an individual one, taking into account the patient's overall health and prior screening history. C recommendation.
Adults in this age group who have never been screened for colorectal cancer are more likely to benefit.
Screening would be most appropriate among adults who (1) are healthy enough to undergo treatment if colorectal cancer is detected and (2) do not have comorbid conditions that would significantly limit their life expectancy.
Rationale
IMPORTANCE
Colorectal cancer is the second-leading cause of cancer death in the United States. In 2016, an estimated 134,000 persons will be diagnosed with the disease, and about 49,000 will die from it. Colorectal cancer is most frequently diagnosed among adults aged 65 to 74 years; the median age at death from colorectal cancer is 68 years.3
DETECTION
The USPSTF found convincing evidence that screening for colorectal cancer with several methods can accurately detect early-stage colorectal cancer and adenomatous polyps.
Although single test performance is an important issue in the detection of colorectal cancer, the sensitivity of the test over time is more important in an ongoing screening program. However, data that permit assessment and direct comparison of screening methods to detect colorectal neoplasia in screening programs over time are limited to those from analytic modeling.
BENEFITS OF SCREENING AND EARLY INTERVENTION
The USPSTF found convincing evidence that screening for colorectal cancer in adults aged 50 to 75 years reduces colorectal cancer mortality. The USPSTF found no head-to-head studies demonstrating that any of the screening strategies it considered are more effective than others, although the tests have varying levels of evidence supporting their effectiveness, as well as different strengths and limitations (Table 2).1,2
About one-third of eligible adults in the United States have never been screened for colorectal cancer,4 and offering choice in colorectal cancer screening strategies may increase screening uptake.5 As such, the screening tests are not presented in any preferred or ranked order; rather, the goal is to maximize the total number of persons who are screened because that will have the largest effect on reducing colorectal cancer deaths.
The benefit of early detection of and intervention for colorectal cancer declines after age 75 years. Among older adults who have been previously screened for colorectal cancer, there is at best a moderate benefit to continuing screening during the ages of 76 to 85 years. However, adults in this age group who have never been screened for colorectal cancer are more likely to benefit than those who have been previously screened.
The time between detection and treatment of colorectal cancer and realization of a subsequent mortality benefit can be substantial. As such, the benefit of early detection of and intervention for colorectal cancer in adults 86 years and older is at most small.
HARMS OF SCREENING AND EARLY INTERVENTION
The harms of screening for colorectal cancer in adults aged 50 to 75 years are small. The majority of harms result from the use of colonoscopy, either as the screening test or as follow-up for positive findings detected by other screening tests. The rate of serious adverse events from colorectal cancer screening increases with age.1 Thus, the harms of screening for colorectal cancer in adults 76 years and older are small to moderate.
USPSTF ASSESSMENT
The USPSTF concludes with high certainty that the net benefit (i.e., the benefit minus the harms) of screening for colorectal cancer in adults aged 50 to 75 years is substantial.
The USPSTF concludes with moderate certainty that the net benefit of screening for colorectal cancer in adults aged 76 to 85 years who have been previously screened is small. Adults who have never been screened for colorectal cancer are more likely to benefit.
Clinical Considerations
PATIENT POPULATION UNDER CONSIDERATION
This recommendation applies to asymptomatic adults 50 years and older who are at average risk of colorectal cancer and who do not have a family history of known genetic disorders that predispose them to a high lifetime risk of colorectal cancer (such as Lynch syndrome or familial adenomatous polyposis), a personal history of inflammatory bowel disease, a previous adenomatous polyp, or previous colorectal cancer.
When screening results in the diagnosis of colorectal adenomas or cancer, patients are followed up with a surveillance regimen, and recommendations for screening no longer apply. The USPSTF did not review or consider the evidence on the effectiveness of any particular surveillance regimen after diagnosis and removal of adenomatous polyps or colorectal cancer.
ASSESSMENT OF RISK
For the vast majority of adults, the most important risk factor for colorectal cancer is older age. Most cases of colorectal cancer occur among adults older than 50 years; the median age at diagnosis is 68 years.3
A positive family history (excluding known inherited familial syndromes) is thought to be linked to about 20% of cases of colorectal cancer.1 About 3% to 10% of the population has a first-degree relative with colorectal cancer.7 The USPSTF did not specifically review the evidence on screening in populations at increased risk; however, other professional organizations recommend that patients with a family history of colorectal cancer (a first-degree relative with early-onset colorectal cancer or multiple first-degree relatives with the disease) be screened more frequently starting at a younger age, and with colonoscopy.8
Male sex and black race are also associated with higher colorectal cancer incidence and mortality. Black adults have the highest incidence and mortality rates compared with other racial/ethnic subgroups.3 The reasons for these disparities are not entirely clear. Studies have documented inequalities in screening, diagnostic follow-up, and treatment; they also suggest that equal treatment generally seems to produce equal outcomes.9–11 Accordingly, this recommendation applies to all racial/ethnic groups, with the clear acknowledgement that efforts are needed to ensure that at-risk populations receive recommended screening, follow-up, and treatment.
SCREENING TESTS
Table 2 lists various screening tests for colorectal cancer and notes potential frequency of use as well as additional considerations for each method.1,2 The figure available on the USPSTF website (https://www.uspreventiveservicestaskforce.org/Page/Document/RecommendationStatementFinal/colorectal-cancer-screening2#fig) presents the estimated number of life-years gained, colorectal cancer deaths averted, lifetime colonoscopies required, and resulting complications per 1,000 screened adults aged 50 to 75 years for each of the screening strategies. These estimates are derived from modeling conducted by the Cancer Intervention and Surveillance Modeling Network (CISNET) to inform this recommendation.2,12
Stool-Based Tests. Multiple randomized clinical trials (RCTs) have shown that screening with the guaiac-based fecal occult blood test (gFOBT) reduces colorectal cancer deaths.1 Fecal immunochemical tests (FITs), which identify intact human hemoglobin in stool, have improved sensitivity compared with gFOBT for detecting colorectal cancer.1 Among the FITs that are cleared by the U.S. Food and Drug Administration (FDA) and available for use in the United States, the OC FIT-CHEK family of FITs (Polymedco)—which include the OC-Light and the OC-Auto—have the best test performance characteristics (i.e., highest sensitivity and specificity).1 Multitargeted stool DNA testing (FIT-DNA) is an emerging screening strategy that combines a FIT with testing for altered DNA biomarkers in cells shed into the stool. Multitargeted stool DNA testing has increased single-test sensitivity for detecting colorectal cancer compared with FIT alone.13 The harms of stool-based testing primarily result from adverse events associated with follow-up colonoscopy of positive findings.1 The specificity of FIT-DNA is lower than that of FIT alone,13 which means it has a higher number of false-positive results and higher likelihood of follow-up colonoscopy and experiencing an associated adverse event per screening test. There are no empirical data on the appropriate longitudinal follow-up for an abnormal FIT-DNA test result followed by a negative colonoscopy; there is potential for overly intensive surveillance due to clinician and patient concerns about the implications of the genetic component of the test.
Direct Visualization Tests. Several RCTs have shown that flexible sigmoidoscopy alone reduces deaths from colorectal cancer.1 Flexible sigmoidoscopy combined with FIT has been studied in a single trial and was found to reduce the colorectal cancer–specific mortality rate more than flexible sigmoidoscopy alone.14 Modeling studies conducted by CISNET also consistently estimate that combined testing yields more life-years gained and colorectal cancer deaths averted compared with flexible sigmoidoscopy alone.2 Flexible sigmoidoscopy can result in direct harms, such as colonic perforations and bleeding, although the associated event rates are much lower than those observed with colonoscopy.1 Harms can also occur as a result of follow-up colonoscopy.
Completed trials of flexible sigmoidoscopy provide indirect evidence that colonoscopy—a similar endoscopic screening method—reduces colorectal cancer mortality. A prospective cohort study also found an association between patients who self-reported being screened with colonoscopy and a lower colorectal cancer mortality rate.15 Colonoscopy has both indirect and direct harms. Harms may be caused by bowel preparation prior to the procedure (e.g., dehydration and electrolyte imbalances), the sedation used during the procedure (e.g., cardiovascular events), or the procedure itself (e.g., infection, colonic perforations, or bleeding).
Evidence for assessing the effectiveness of computed tomography (CT) colonography is limited to studies of its test characteristics.1 Computed tomography colonography can result in unnecessary diagnostic testing or treatment of incidental extracolonic findings that are of no importance or would never have threatened the patient's health or become apparent without screening (i.e., over-diagnosis and overtreatment).1 Extracolonic findings are common, occurring in about 40% to 70% of screening examinations. Between 5% and 37% of these findings result in diagnostic follow-up, and about 3% require definitive treatment.1 As with other screening strategies, indirect harms from CT colonography can also occur from follow-up colonoscopy for positive findings.
Serology Tests. The FDA approved a blood test to detect circulating methylated SEPT9 DNA (Epi proColon; Epigenomics) in April 2016.16 A single test characteristic study met the inclusion criteria for the systematic evidence review supporting this recommendation statement; it found the SEPT9 DNA test to have low sensitivity (48%) for detecting colorectal cancer.17
STARTING AND STOPPING AGES
Available RCTs of gFOBT and flexible sigmoidoscopy included patients with age ranges of 45 to 80 years and 50 to 74 years, respectively. For gFOBT, the majority of participants entered the trials at age 50 or 60 years; for flexible sigmoidoscopy, the mean age of participants was 56 to 60 years.1
Microsimulation analyses performed by CISNET suggest that starting colorectal cancer screening at age 45 years rather than 50 years is estimated to yield a modest increase in life-years gained and a more efficient balance between life-years gained and lifetime number of colonoscopies (a proxy measure for the burden of screening).2 However, across the different screening methods, lowering the age at which to begin screening to 45 years while maintaining the same screening interval resulted in an estimated increase in the lifetime number of colonoscopies. In the case of screening colonoscopy, 2 of the 3 models found that by starting screening at age 45 years, the screening interval could be extended from 10 to 15 years. Doing so maintained the same (or slightly more) life-years gained as performing colonoscopy every 10 years starting at age 50 years without increasing the lifetime number of colonoscopies. However, 1 model estimated a slight loss in life-years gained with a longer screening interval and an earlier age at which to begin screening.2
The USPSTF considered these findings and concluded that the evidence best supports a starting age of 50 years for the general population, noting the modest increase in life-years gained by starting screening earlier, the discordant findings across models for extending the screening interval when the age at which to begin screening is lowered, and the lack of empirical evidence in younger populations.
The age at which the balance of benefits and harms of colorectal cancer screening becomes less favorable varies based on a patient's life expectancy, health status, comorbid conditions, and prior screening status.18 Empirical data from randomized trials on outcomes of screening after age 74 years are scarce. All 3 CISNET models consistently estimate that few additional life-years are gained when screening is extended past age 75 years among average-risk adults who have previously received adequate screening.2
The USPSTF does not recommend routine screening for colorectal cancer in adults 86 years and older. In this age group, competing causes of mortality preclude a mortality benefit that would outweigh the harms.
SCREENING INTERVALS
Evidence from RCTs demonstrates that annual or biennial screening with gFOBT as well as 1-time and every 3- to 5-year flexible sigmoidoscopy reduces colorectal cancer deaths.1 The CISNET models found that several screening strategies were estimated to yield comparable life-years gained (i.e., life-years gained with the non-colonoscopy strategies were within 90% of those gained with the colonoscopy strategy) among adults aged 50 to 75 years and an efficient balance of benefits and harms (see the full CISNET report for more details2,12). These screening strategies include (1) annual screening with FIT, (2) screening every 10 years with flexible sigmoidoscopy and annual screening with FIT, (3) screening every 10 years with colonoscopy, and (4) screening every 5 years with CT colonography. The findings for CT colonography depend on the proxy measure used for the burden of screening (number of lifetime colonoscopies or lifetime cathartic bowel preparations). Two of the 3 CISNET models found that FIT-DNA screening every 3 years (as recommended by the manufacturer) was estimated to yield life-years gained less than 90% of the colonoscopy screening strategy (84% and 87%, respectively). Another way to conceptualize these findings is to note that CISNET modeling found that FIT-DNA screening every 3 years was estimated to provide about the same amount of benefit as screening with flexible sigmoidoscopy alone every 5 years (https://www.uspreventiveservicestaskforce.org/Page/Document/RecommendationStatementFinal/colorectal-cancer-screening2#fig).2
TREATMENT
Treatment of early-stage colorectal cancer generally consists of local excision or simple polypectomy for tumors limited to the colonic mucosa or surgical resection (via laparoscopy or open approach) with anastomosis for larger, localized lesions.
OTHER APPROACHES TO PREVENTION
The USPSTF has made a recommendation on aspirin use for the primary prevention of cardiovascular disease and colorectal cancer in average-risk adults (http://www.uspreventiveservicestaskforce.org).
This recommendation statement was first published in JAMA. 2016;315 (23):2564–2575 [published correction appears in JAMA. 2016;316(5):545].
The “Other Considerations,” “Discussion,” “Update of Previous USPSTF Recommendation,” and “Recommendations of Others” sections of this recommendation statement are available at https://www.uspreventiveservicestaskforce.org/Page/Document/UpdateSummaryFinal/colorectal-cancer-screening2.
The USPSTF recommendations are independent of the U.S. government. They do not represent the views of the Agency for Healthcare Research and Quality, the U.S. Department of Health and Human Services, or the U.S. Public Health Service.