A more recent article on deep venous thrombosis and pulmonary embolism is available.
This is a corrected version of the article that appeared in print.
Am Fam Physician. 2017;95(5):295-302
Related letters: Clarification for Apixaban Dosing in Patients with Impaired Renal Function and Treatment of Venous Thromboembolism in Patients Who Are Morbidly Obese
Author disclosure: No relevant financial affiliations.
Pulmonary embolism and deep venous thrombosis are the two most important manifestations of venous thrombo-embolism (VTE), which is the third most common life-threatening cardiovascular disease in the United States. Anticoagulation is the mainstay of VTE treatment. Most patients with deep venous thrombosis or low-risk pulmonary embolism can be treated in the outpatient setting with low-molecular-weight heparin and a vitamin K antagonist (warfarin) or direct-acting oral anticoagulants. Inpatient treatment of VTE begins with parenteral agents, preferably low-molecular-weight heparin. Unfractionated heparin is used if a patient is hemodynamically unstable or has severe renal insufficiency, high bleeding risk, hemodynamic instability, or morbid obesity. Direct-acting oral anticoagulants are an alternative; however, concerns include cost and use of reversing agents (currently available only for dabigatran, although others are in development). If warfarin is used, low-molecular-weight or unfractionated heparin must be administered concomitantly for at least five days or until the international normalized ratio becomes therapeutic for 24 hours. Dabigatran or edoxaban should be initiated after five to 10 days of initial therapy with a parenteral anticoagulant. [corrected] Hemodynamically unstable patients with a low bleeding risk may benefit from thrombolytic therapy. An inferior vena cava filter is not indicated for patients treated with anticoagulation. Current guidelines recommend anticoagulation for a minimum of three months. Special situations, such as active cancer and pregnancy, require long-term use of low-molecular-weight or unfractionated heparin. Anticoagulation beyond three months should be individualized based on a risk/benefit analysis. Symptomatic distal deep venous thrombosis should be treated with anticoagulation, but asymptomatic patients may be monitored with serial imaging for two weeks and treated only if there is extension.
Deep venous thrombosis (DVT) and pulmonary embolism (PE) are the two most important manifestations of venous thromboembolism (VTE), which is the third most common life-threatening cardiovascular disease, after myocardial infarction and stroke, in the United States.1 According to the Centers for Disease Control and Prevention, the annual incidence of VTE is one or two per 1,000 persons, and the overall mortality rate is between 60,000 and 100,000 annually.2 One-half of patients with DVT will have long-term complications, including postthrombotic syndrome and venous ulcers. One-third of patients with VTE will have a recurrence within 10 years.2
| Clinical recommendation | Evidence rating | References |
|---|---|---|
| Direct-acting oral anticoagulants are an alternative to vitamin K antagonist therapy (warfarin [Coumadin]) for VTE. | A | 4, 19, 20 |
| Most patients with deep venous thrombosis and selected patients with pulmonary embolism can be safely treated as outpatients. | B | 8, 10, 11 |
| Inferior vena cava filters should be avoided in patients with VTE treated with anticoagulation. | B | 8, 9, 26, 27 |
| If there are no contraindications, patients diagnosed with acute VTE should receive anticoagulation for a minimum of three months. | C | 8, 9, 29 |

| Recommendation | Sponsoring organization |
|---|---|
| Do not recommend bed rest following diagnosis of acute deep venous thromboembolism after the initiation of anticoagulation therapy, unless significant medical concerns are present. | American Physical Therapy Association |
| Do not treat with an anticoagulant for more than three months in a patient with a first venous thromboembolism occurring in the setting of a major transient risk factor. | American Society of Hematology |
Approximately one-third of patients with VTE present with PE, and two-thirds present with DVT.1 Compared with DVT, PE is more often fatal, has a higher recurrence rate, and is associated with more serious long-term complications. Of patients with proximal DVT, 40% have an associated PE, whereas 70% of patients with PE also have DVT.3
Similarities in pathogenesis between DVT and PE parallel the similarities in their management, including anticoagulation, risk factor assessment, and perioperative management. For decades, DVT and PE have been treated with unfractionated or low-molecular-weight heparin and the vitamin K antagonist warfarin (Coumadin). Direct-acting oral anticoagulants are a safe and effective alternative to warfarin that are supported by published guidelines.4,5
Initial Management
Prompt diagnosis and treatment of VTE with appropriate medications may prevent thrombus extension and embolization, relieve acute symptoms, prevent cardiopulmonary collapse, and reduce the risk of long-term complications. Empiric treatment during the evaluation period is controversial and not evidence-based. In a hemodynamically unstable patient with a high probability of VTE, intravenous thrombolytic therapy can be considered.9 Similarly, if there is a delay in obtaining a definitive diagnostic test in a hemodynamically unstable patient with a high probability of VTE, parenteral anticoagulation should be considered until a diagnosis is confirmed.
After diagnosis, most patients with DVT can be treated as an outpatient, except in cases of limb ischemia, significant comorbidities (e.g., end-stage renal disease), functional limitations, high bleeding risk, or nonadherence concerns. Anticoagulation is not recommended for isolated distal DVTs (i.e., confined to calf veins) unless the patient is symptomatic, has risk factors for extension (e.g., unprovoked DVT, prior VTE), or develops extension of DVT on serial imaging for two weeks.8,10,11
Evidence supports outpatient treatment of PE if the risk of nonadherence is low and the patient is clinically stable; has no contraindications to anticoagulation, such as recent bleeding, severe renal or liver disease, or platelet count of less than 70 × 103 per mm3 (70 × 109 per L); and feels capable of managing the disease at home.8,10 Patients with PE who are hemodynamically unstable (e.g., those with hypotension or evidence of shock) should be admitted to an intensive care unit, and systemic thrombolytic therapy may be considered.9
Anticoagulation Choices
Once VTE is diagnosed and the patient is stabilized if needed, anticoagulation should be initiated unless contraindicated. Guideline recommendations for anticoagulation are divided into phases: initial phase (first week after diagnosis), long-term phase (second week to three months), and extended phase (beyond three months).9
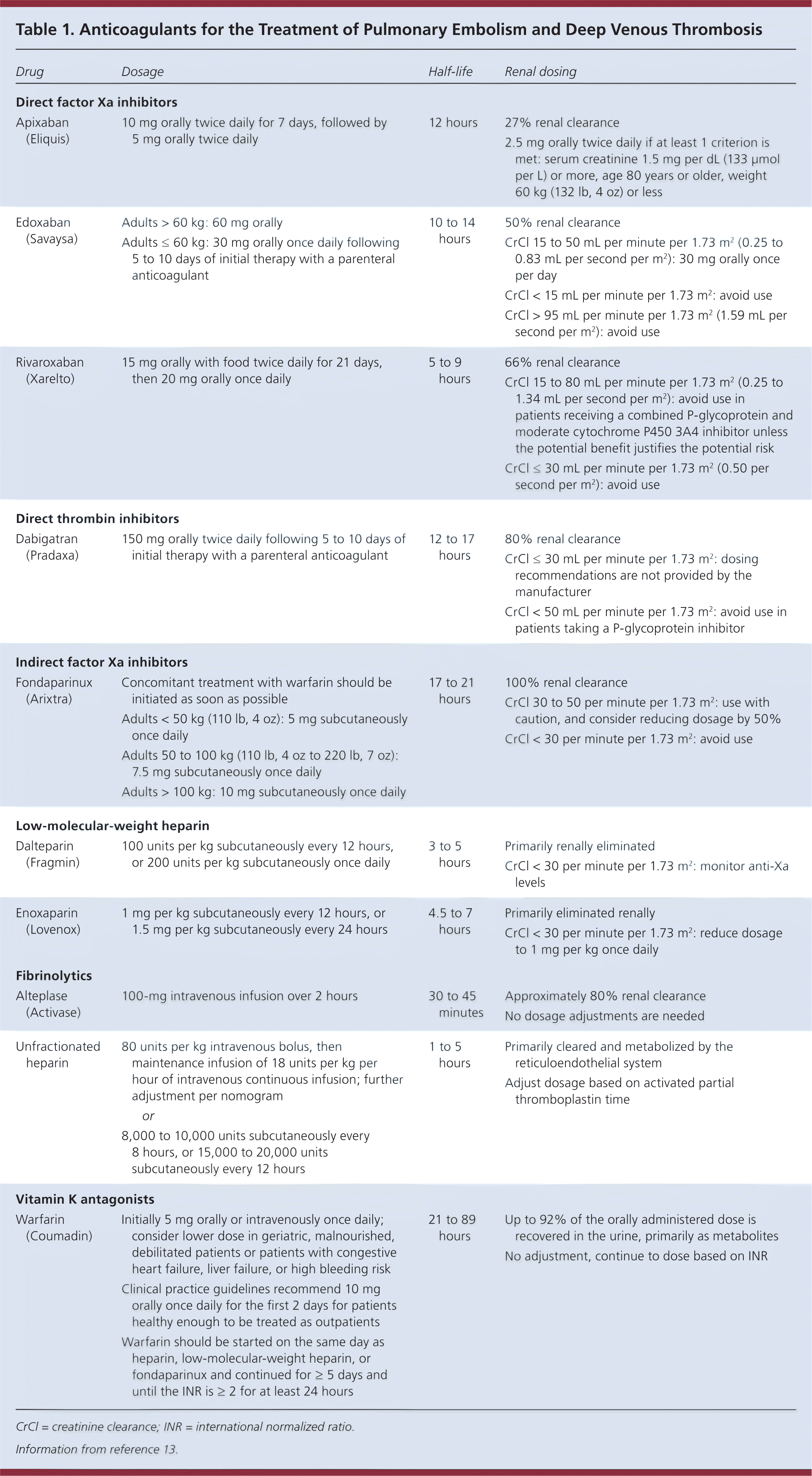
In the initial phase of anticoagulation, a decision must be made between using the vitamin K antagonist warfarin or a direct-acting oral anticoagulant. If warfarin is selected, concomitant parenteral anticoagulation is required for at least five days; if dabigatran (Pradaxa) or edoxaban (Savaysa) is selected they should be initiated after five to 10 days of initial therapy with a parenteral anticoagulant. [corrected] Guidelines recommend low-molecular-weight over unfractionated heparin, which is supported by multiple therapeutic trials showing greater effectiveness and safety and lower mortality.12 However, unfractionated heparin is preferred in patients with severe renal insufficiency, high bleeding risk, hemodynamic instability, or morbid obesity.7–9 Apixaban (Eliquis) and rivaroxaban (Xarelto) do not require concomitant use of heparin at initiation. The administration and dosing of anticoagulants are included in Table 1.13 [corrected]
| Drug | Dosage | Half-life | Renal dosing |
|---|---|---|---|
| Direct factor Xa inhibitors | |||
|
|
|
|
|
|
|
|
|
|
|
|
| Direct thrombin inhibitors | |||
|
|
|
|
| Indirect factor Xa inhibitors | |||
|
|
|
|
| Low-molecular-weight heparin | |||
|
|
|
|
|
|
|
|
| Fibrinolytics | |||
|
|
|
|
|
|
|
|
| Vitamin K antagonists | |||
|
|
|
|
If a patient is admitted, discharge should occur when the patient has clinically improved and is hemodynamically stable. The initial treatment phase (first week) can be completed in the outpatient setting after thorough patient education on anticoagulation therapy. During the initial three months of anticoagulation, patients should be evaluated periodically for adherence and complications of treatment, especially bleeding. Frequency of physician visits is individualized based on patient knowledge and adherence, and on which therapy is selected.
If warfarin is used, patients require careful education on food and drug interactions and the importance of regular office visits to check international normalized ratio until a steady state is achieved. For selected patients, physician-directed, home-based international normalized ratio monitoring provides a convenient alternative to office visits.6
Direct-Acting Oral Anticoagulants
In 2012, rivaroxaban became the first direct-acting oral anticoagulant approved by the U.S. Food and Drug Administration for treatment of DVT and PE. Several others followed. These agents belong to two classes: direct thrombin inhibitors (dabigatran) and direct factor Xa inhibitors (apixaban, edoxaban, and rivaroxaban).14–17
There are logistic benefits of direct-acting anticoagulants compared with warfarin—primarily that no regular monitoring is required because of their predictable pharmacokinetics. Other benefits compared with warfarin include fewer dietary restrictions, fewer drug interactions, and relatively fixed dosing. Rivaroxaban should be taken with food, and it interacts with cytochrome P450 3A4 and P-glycoprotein inhibitors. Dabigatran may be affected by P-glycoprotein inducers or inhibitors. Dose adjustment may be required for these medications.4
The drawbacks of direct-acting anticoagulants are cost ($349 to $430 per month, U.S. average wholesale price 18) and uncertainties regarding management of major bleeding or emergent surgery. Direct-acting anticoagulants have shorter half-lives than warfarin, and missed doses or premature discontinuation increases the risk of thrombotic events. Also, because elimination of direct-acting anticoagulants is more dependent on renal function than with warfarin, dose adjustment may be required for patients with chronic kidney disease.19,20 The initial studies of direct-acting anticoagulants excluded several important patient populations, such as pregnant women, patients with an active cancer diagnosis, and patients who are morbidly obese; thus, there are no data to guide therapy in these groups.19,20
Only dabigatran has a commercially available reversal agent, although other reversal agents are in development. In 2015, the U.S. Food and Drug Administration approved idarucizumab (Praxbind), a monoclonal antibody that binds dabigatran in the serum.21 In instances of major bleeding, there are case series and expert recommendations to guide interventions. For patients experiencing a devastating bleed, such as intracranial hemorrhage, treatment includes stopping the direct-acting anticoagulant; initiating supportive therapy; and administering activated charcoal, antifibrinolytic agents, and prothrombin complex concentrate.22 Hemodialysis should be considered for severe cases in patients taking dabigatran, but it is not effective for patients taking factor Xa inhibitors.
The ACCP recommends the use of direct-acting anticoagulants over warfarin for VTE treatment in patients without cancer (weak recommendation based on moderate quality evidence, per the ACCP grading system).8 For patients with recurrent VTE who are already taking an oral anticoagulant, low-molecular-weight heparin is recommended over other oral anticoagulants. For patients with recurrent VTE while taking a low-molecular-weight heparin, the dose should be increased by 25% to 33% (weak recommendations based on moderate to poor quality evidence per the ACCP grading system).8
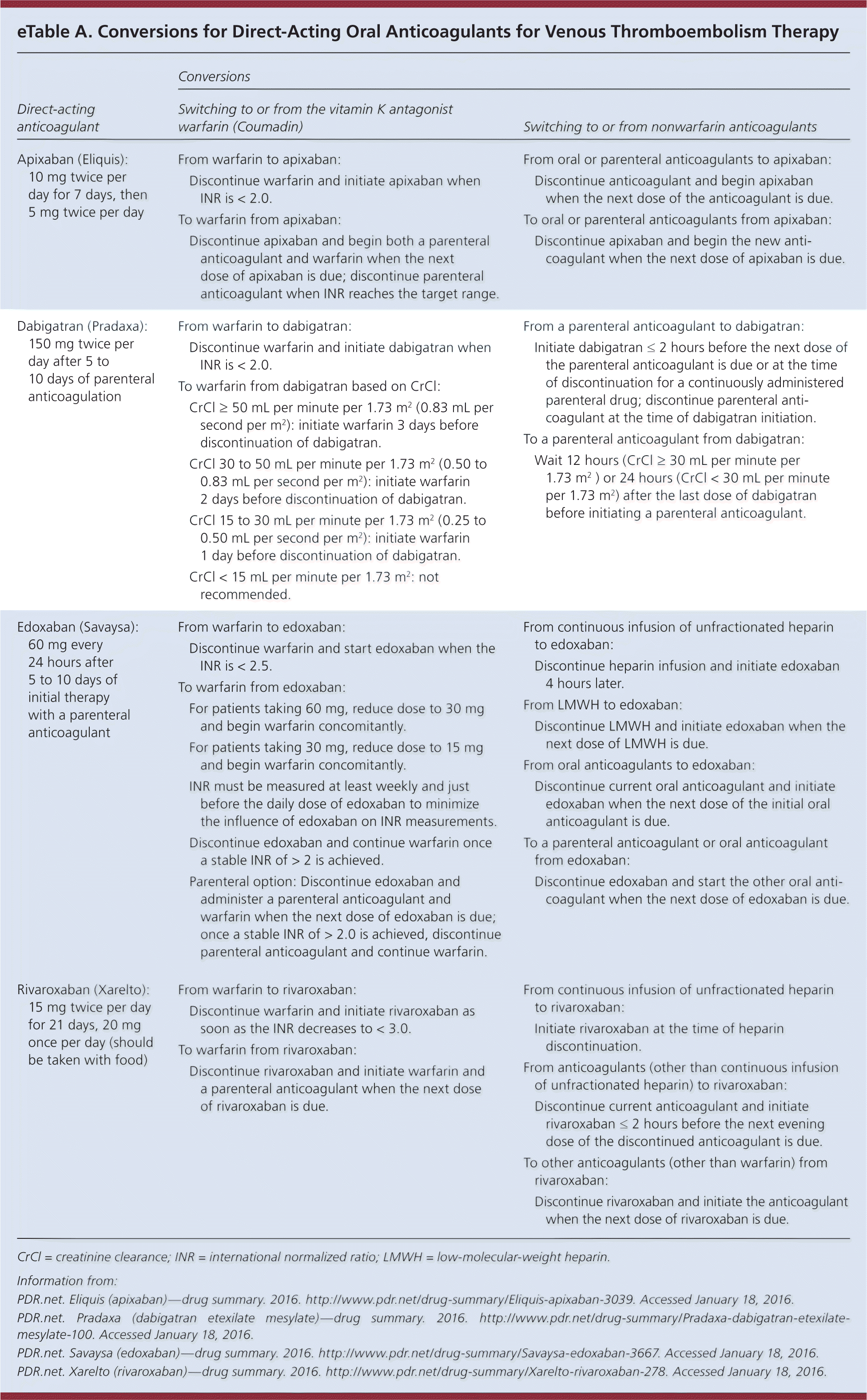
For patients transitioning from one anticoagulant to another, specific recommendations for conversion are available in eTable A and from the U.S. Food and Drug Administration's approved drugs section (http://www.accessdata.fda.gov/scripts/cder/daf/).
| Conversions | ||||
|---|---|---|---|---|
| Direct-acting anticoagulant | Switching to or from the vitamin K antagonist warfarin (Coumadin) | Switching to or from nonwarfarin anticoagulants | ||
|
|
| ||
|
|
| ||
|
|
| ||
|
|
| ||
Thrombolysis
Because of the high risk of bleeding, thrombolysis is restricted to specific circumstances. Expert consensus guidelines support thrombolytic therapy in patients with persistent hypotension or shock secondary to acute PE.9 Also, when patients with acute PE who are on anticoagulation deteriorate but are not yet hypotensive, systemic thrombolysis is recommended as long as the risk of bleeding is low.8 There is better evidence for systemic thrombolysis than for catheter-directed thrombolysis.8 If systemic thrombolysis fails, catheter-directed thrombolysis is available as a rescue therapy in centers with appropriate expertise. Thrombolysis is not indicated in hemodynamically stable patients with intermediate-risk PE.23
Massive proximal lower extremity thrombosis or ilio-femoral thrombosis associated with severe symptoms or limb-threatening ischemia for less than 14 days is the only widely accepted indication for thrombolytic therapy in patients with DVT.9 Treatment modalities include systemic thrombolysis, catheter-directed thrombolysis, and surgical thrombectomy. The most appropriate therapy depends on the treatment center's expertise.
Inferior Vena Cava Filters
An inferior vena cava filter is rarely indicated, and evidence for safety and effectiveness is lacking.24 If there is an absolute contraindication to therapeutic anticoagulation, complications from anticoagulation, or failure of anticoagulation in a patient with acute proximal DVT, an inferior vena cava filter may be indicated. Its use for other reasons is controversial.25 Routine use of inferior vena cava filters in patients on anticoagulation does not reduce mortality, even in high-risk patients,26 and current guidelines recommend against their use in these patients.8,9 Possible complications from inferior vena cava filter placement include thrombosis and arteriovenous fistula. Retrieval is difficult and has a failure rate of at least 8%.27
Treatment Duration
The risk of VTE recurrence is greatest in the first year after the event and remains elevated indefinitely compared with the general population. Lifetime recurrence rates for DVT ranges from 21% to 30%, depending on the population.10,28 Risk of VTE is increased by patient factors, such as active cancer and thrombophilia.
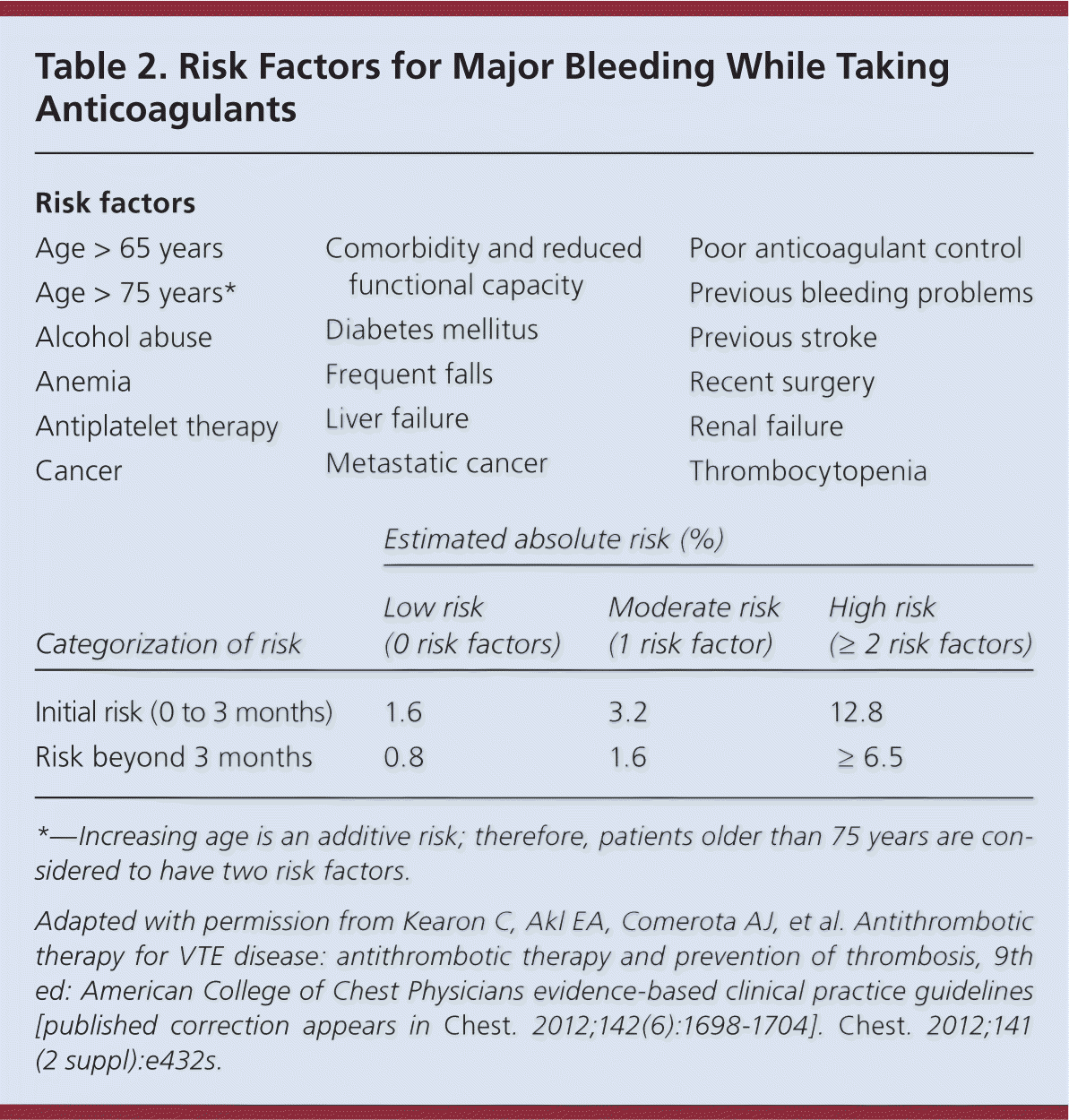
Long-term anticoagulation reduces the risk of recurrent VTE but results in more bleeding events. Considering this trade-off, it is critical that the duration of anticoagulation therapy be individualized based on the patient's risk of recurrence vs. risk of bleeding. Risk factors for bleeding are summarized in Table 2.9 If there are no contraindications, current guidelines recommend anticoagulation for a minimum of three months for PE and proximal DVT.8,29 If a reversible provoking factor is identified as the cause of VTE, anticoagulation beyond three months is not recommended.7–9 Extended anticoagulation is recommended for patients with an unprovoked VTE and low risk of bleeding.8,9 Indefinite anticoagulation is recommended for patients with a second VTE and low or moderate risk of bleeding.8,9
| Risk factors | ||
|---|---|---|
| Age > 65 years | Comorbidity and reduced functional capacity | Previous bleeding problems |
| Age > 75 years* | Diabetes mellitus | Previous stroke |
| Alcohol abuse | Frequent falls | Recent surgery |
| Anemia | Liver failure | Renal failure |
| Antiplatelet therapy | Metastatic cancer | Thrombocytopenia |
| Cancer | Poor anticoagulant control | |
The d-dimer test has been used to stratify risk of recurrent VTE. The d-dimer value is checked one month after anticoagulation ends, with an increased level indicating increased risk.30 However, the ACCP does not recommend its routine use to determine appropriate candidates for indefinite anticoagulation.8
Special Considerations
Some conditions, such as pregnancy and cancer, require special consideration when treating VTE. These special considerations are described in Table 3.6,8,9,31–39 The ACCP does not recommend routine use of compression stockings to prevent postthrombotic syndrome. For patients with low-risk subsegmental PE without proximal DVT, clinical surveillance is preferred over anticoagulation.8
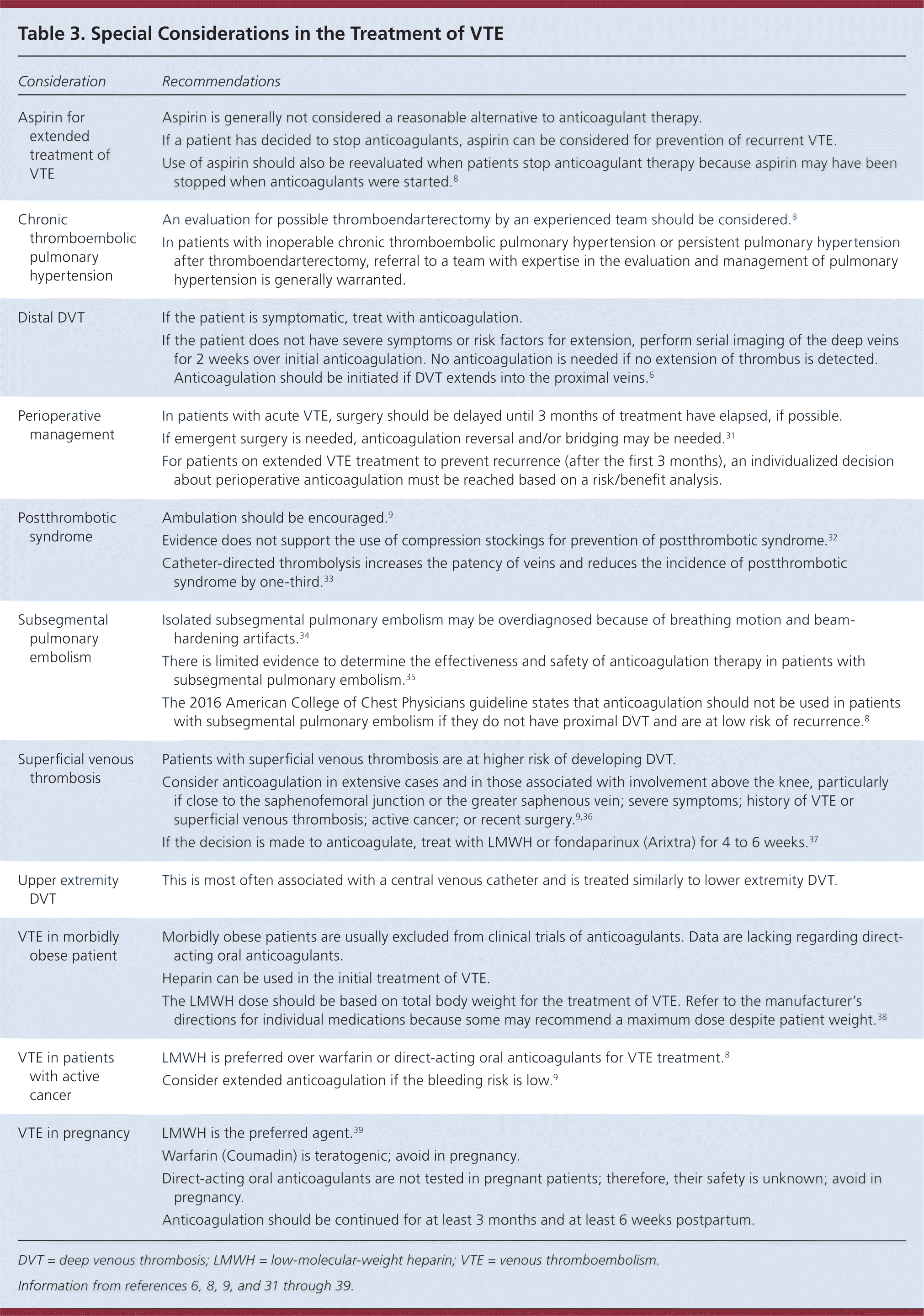
| Consideration | Recommendations |
|---|---|
| Aspirin for extended treatment of VTE | Aspirin is generally not considered a reasonable alternative to anticoagulant therapy. |
| If a patient has decided to stop anticoagulants, aspirin can be considered for prevention of recurrent VTE. | |
| Use of aspirin should also be reevaluated when patients stop anticoagulant therapy because aspirin may have been stopped when anticoagulants were started.8 | |
| Chronic thromboembolic pulmonary hypertension | An evaluation for possible thromboendarterectomy by an experienced team should be considered.8 |
| In patients with inoperable chronic thromboembolic pulmonary hypertension or persistent pulmonary hypertension after thromboendarterectomy, referral to a team with expertise in the evaluation and management of pulmonary hypertension is generally warranted. | |
| Distal DVT | If the patient is symptomatic, treat with anticoagulation. |
| If the patient does not have severe symptoms or risk factors for extension, perform serial imaging of the deep veins for 2 weeks over initial anticoagulation. No anticoagulation is needed if no extension of thrombus is detected. Anticoagulation should be initiated if DVT extends into the proximal veins.6 | |
| Perioperative management | In patients with acute VTE, surgery should be delayed until 3 months of treatment have elapsed, if possible. |
| If emergent surgery is needed, anticoagulation reversal and/or bridging may be needed.31 | |
| For patients on extended VTE treatment to prevent recurrence (after the first 3 months), an individualized decision about perioperative anticoagulation must be reached based on a risk/benefit analysis. | |
| Postthrombotic syndrome | Ambulation should be encouraged.9 |
| Evidence does not support the use of compression stockings for prevention of postthrombotic syndrome.32 | |
| Catheter-directed thrombolysis increases the patency of veins and reduces the incidence of postthrombotic syndrome by one-third.33 | |
| Subsegmental pulmonary embolism | Isolated subsegmental pulmonary embolism may be overdiagnosed because of breathing motion and beam-hardening artifacts.34 |
| There is limited evidence to determine the effectiveness and safety of anticoagulation therapy in patients with subsegmental pulmonary embolism.35 | |
| The 2016 American College of Chest Physicians guideline states that anticoagulation should not be used in patients with subsegmental pulmonary embolism if they do not have proximal DVT and are at low risk of recurrence.8 | |
| Superficial venous thrombosis | Patients with superficial venous thrombosis are at higher risk of developing DVT. |
| Consider anticoagulation in extensive cases and in those associated with involvement above the knee, particularly if close to the saphenofemoral junction or the greater saphenous vein; severe symptoms; history of VTE or superficial venous thrombosis; active cancer; or recent surgery.9,36 | |
| If the decision is made to anticoagulate, treat with LMWH or fondaparinux (Arixtra) for 4 to 6 weeks.37 | |
| Upper extremity DVT | This is most often associated with a central venous catheter and is treated similarly to lower extremity DVT. |
| VTE in morbidly obese patient | Morbidly obese patients are usually excluded from clinical trials of anticoagulants. Data are lacking regarding direct-acting oral anticoagulants. |
| Heparin can be used in the initial treatment of VTE. | |
| The LMWH dose should be based on total body weight for the treatment of VTE. Refer to the manufacturer's directions for individual medications because some may recommend a maximum dose despite patient weight.38 | |
| VTE in patients with active cancer | LMWH is preferred over warfarin or direct-acting oral anticoagulants for VTE treatment.8 |
| Consider extended anticoagulation if the bleeding risk is low.9 | |
| VTE in pregnancy | LMWH is the preferred agent.39 |
| Warfarin (Coumadin) is teratogenic; avoid in pregnancy. | |
| Direct-acting oral anticoagulants are not tested in pregnant patients; therefore, their safety is unknown; avoid in pregnancy. | |
| Anticoagulation should be continued for at least 3 months and at least 6 weeks postpartum. |
Data Sources: We searched PubMed, Cochrane database, Essential Evidence, and guidelines.gov using the following terms in various combinations: management, treatment, therapy, venous thromboembolism, deep venous thrombosis, pulmonary embolus, antithrombotic therapy, direct-acting oral anticoagulants, and new oral anticoagulants. Search dates: September 2015 to February 2016, and December 10, 2016.