Am Fam Physician. 2017;95(5):324-326
Author disclosure: No relevant financial affiliations.
Key Clinical Issue
What are the benefits and harms of treatments for adults with binge-eating disorder?
Evidence-Based Answer
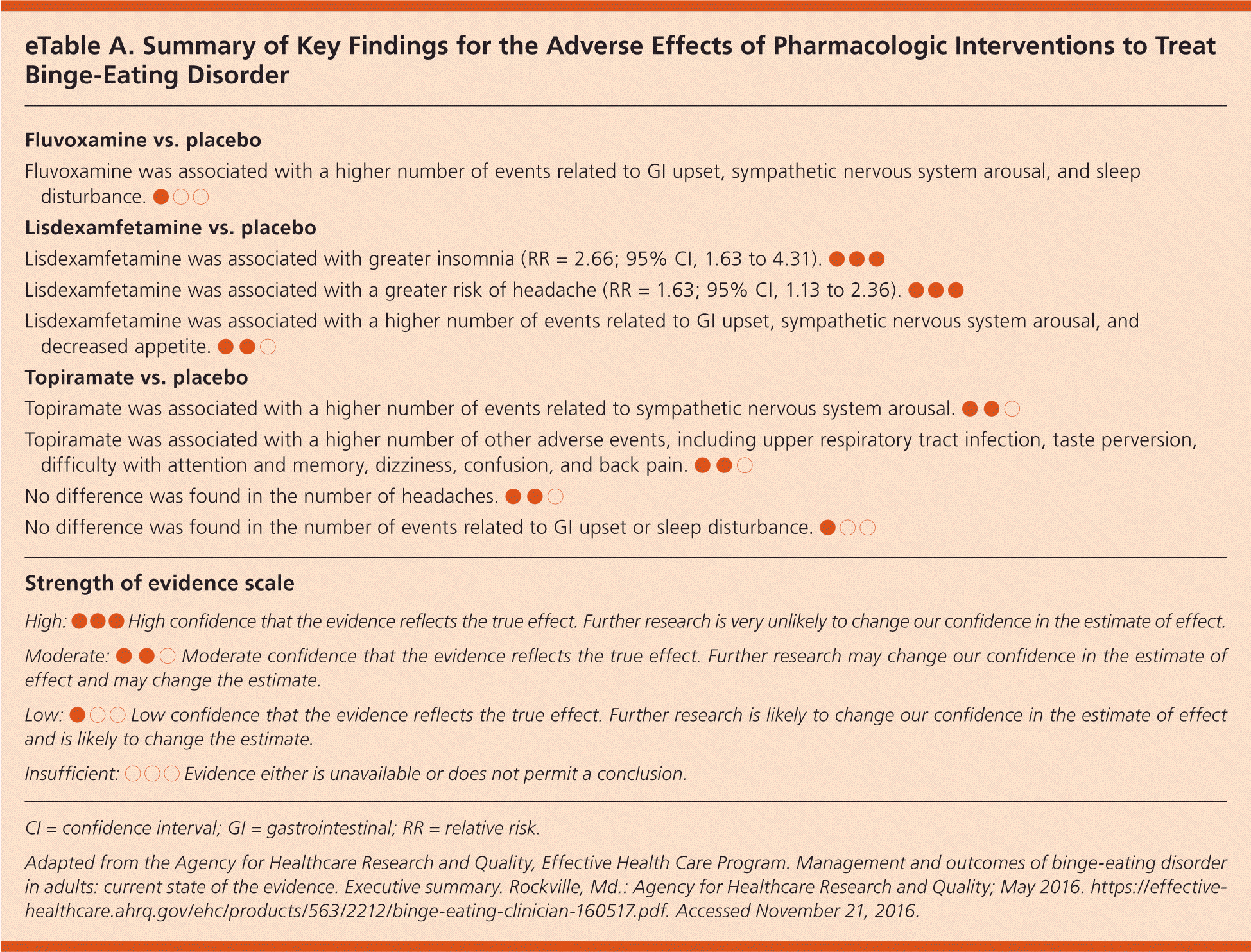
Therapist-led cognitive behavior therapy (CBT) reduces binge-eating frequency and increases binge-eating abstinence. (Strength of Recommendation [SOR]: A, based on consistent, good-quality patient-oriented evidence.) In short-term studies (six to 16 weeks), lisdexamfetamine, second-generation antidepressants, and topira-mate increased binge-eating abstinence and reduced binge-eating frequency and eating-related obsessions and compulsions. (SOR: B, based on inconsistent or limited-quality patient-oriented evidence.) Adverse effects of pharmacologic interventions were rarely severe (eTable A).
| Fluvoxamine vs. placebo |
| Fluvoxamine was associated with a higher number of events related to GI upset, sympathetic nervous system arousal, and sleep disturbance. ●○○ |
| Lisdexamfetamine vs. placebo |
| Lisdexamfetamine was associated with greater insomnia (RR = 2.66; 95% CI, 1.63 to 4.31). ●●● |
| Lisdexamfetamine was associated with a greater risk of headache (RR = 1.63; 95% CI, 1.13 to 2.36). ●●● |
| Lisdexamfetamine was associated with a higher number of events related to GI upset, sympathetic nervous system arousal, and decreased appetite. ●●○ |
| Topiramate vs. placebo |
| Topiramate was associated with a higher number of events related to sympathetic nervous system arousal. ●●○ |
| Topiramate was associated with a higher number of other adverse events, including upper respiratory tract infection, taste perversion, difficulty with attention and memory, dizziness, confusion, and back pain. ●●○ |
| No difference was found in the number of headaches. ●●○ |
| No difference was found in the number of events related to GI upset or sleep disturbance. ●○○ |

| Psychological and behavior therapy interventions | |
| Therapist-led CBT vs. wait-list* | |
| CBT increased binge-eating abstinence (RR = 4.95; 95% CI, 3.06 to 8.00). ●●● | |
| CBT decreased the frequency of binge-eating episodes per week (MD = −2.32; 95% CI, −4.56 to −0.09). ●●● | |
| CBT decreased eating-related psychopathology. ●●● | |
| No differences were found in body mass index or symptoms of depression. ●●○ | |
| Therapist-led CBT vs. behavioral weight loss therapy | |
| Behavioral weight loss therapy decreased body mass index more than CBT at the end of treatment. ●●○ | |
| CBT decreased binge-eating frequency more than behavioral weight loss therapy at the end of treatment and for up to 12 months of follow-up. ●○○ | |
| No differences were found in binge-eating abstinence or symptoms of depression. ●○○ | |
| Pharmacologic interventions | |
| Lisdexamfetamine† (a central nervous system stimulant) vs. placebo | |
| Lisdexamfetamine increased binge-eating abstinence (RR = 2.61; 95% CI, 2.04 to 3.33). ●●● | |
| Lisdexamfetamine decreased binge-eating days per week, weight, and eating-related obsessions and compulsions. ●●● | |
| Second-generation antidepressants (as a class) vs. placebo | |
| Antidepressants increased binge-eating abstinence (RR = 1.67; 95% CI, 1.24 to 2.26) and decreased the frequency of binge-eating episodes per week (MD = −0.67; 95% CI, −1.26 to −0.09). ●●● | |
| Antidepressants decreased the frequency of binge-eating days per week (MD = −0.90; 95% CI, −1.48 to −0.32) and eating-related obsessions and compulsions. ●●○ | |
| Antidepressants decreased symptoms of depression (MD = −1.98; 95% CI, −3.67 to −0.28) ●○○ | |
| No differences were found in weight or body mass index. ●○○ | |
| Topiramate (an anticonvulsant) vs. placebo | |
| Topiramate increased binge-eating abstinence and decreased binge-eating frequency, weight, and eating-related obsessions and compulsions. ●●○ | |
| Topiramate improved general and eating-related psychological functioning, and decreased impulsivity and disability in family and other social domains. ●○○ | |
Practice Pointers
Binge-eating disorder is characterized by recurrent episodes of eating unusually large portions in a discrete period (two hours or less), at least once a week for three months. Core features include lack of control over eating, eating rapidly, feeling uncomfortably full, eating alone, and feeling guilty or depressed. Binge-eating disorder is not associated with inappropriate compensatory behaviors (e.g., purging).1 The lifetime prevalence in the United States is 2.8%. It is more common in females, adolescents, and obese individuals. Severity is characterized by the number of episodes per week.2
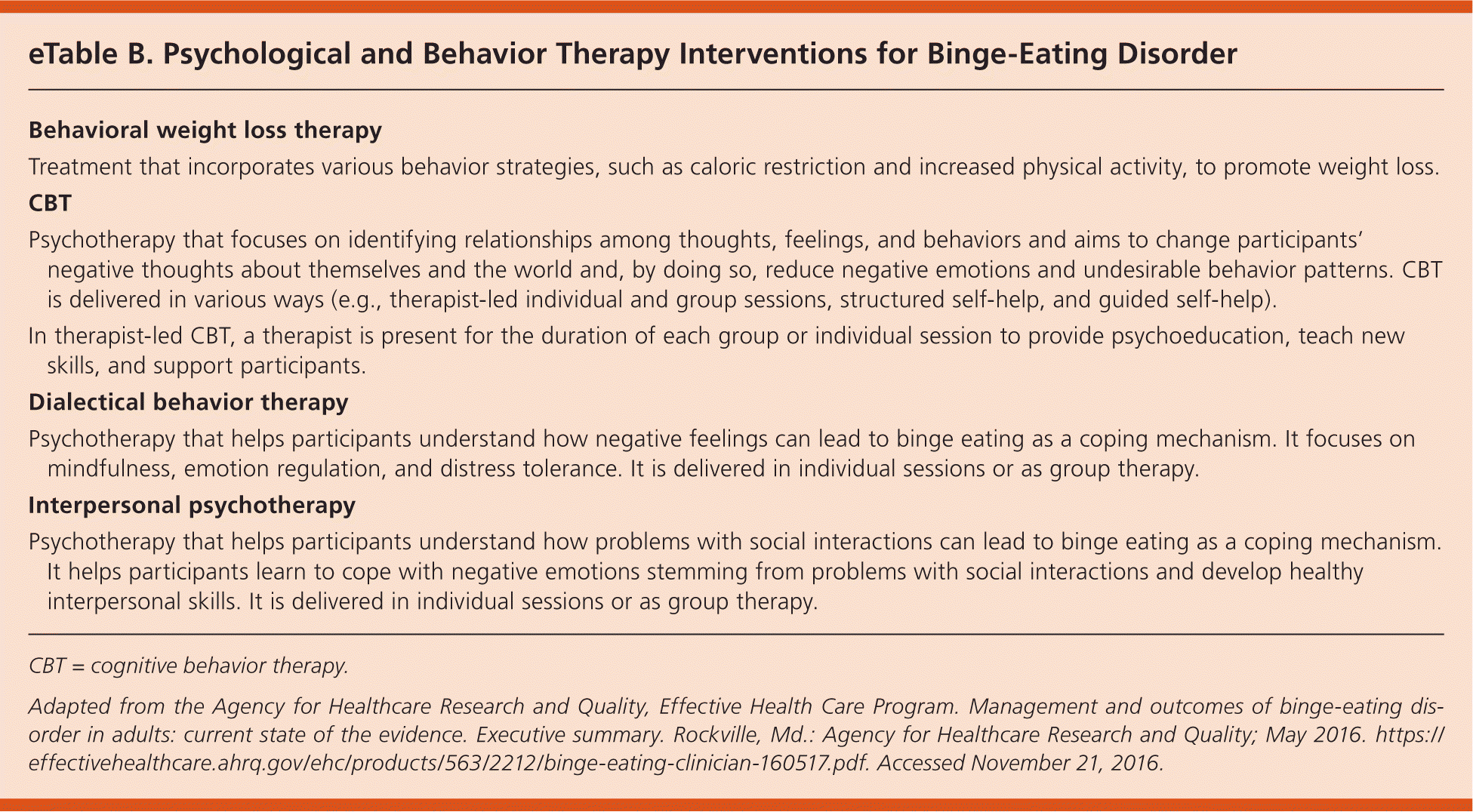
Binge-eating disorder can be treated with psychological and behavior therapies (eTable B) and pharmacotherapy. Psychotherapeutic options include behavioral weight loss therapy, CBT, interpersonal psychotherapy, and dialectical behavior therapy. CBT focuses on changing negative thoughts and undesirable behaviors.3 It can be therapist-led (individual or group sessions), guided self-help, or structured self-help. The U.S. Food and Drug Administration approved lisdexamfetamine for treatment of moderate to severe binge-eating disorder. Other treatments include topiramate and second-generation antidepressants.4
| Behavioral weight loss therapy |
| Treatment that incorporates various behavior strategies, such as caloric restriction and increased physical activity, to promote weight loss. |
| CBT |
| Psychotherapy that focuses on identifying relationships among thoughts, feelings, and behaviors and aims to change participants' negative thoughts about themselves and the world and, by doing so, reduce negative emotions and undesirable behavior patterns. CBT is delivered in various ways (e.g., therapist-led individual and group sessions, structured self-help, and guided self-help). |
| In therapist-led CBT, a therapist is present for the duration of each group or individual session to provide psychoeducation, teach new skills, and support participants. |
| Dialectical behavior therapy |
| Psychotherapy that helps participants understand how negative feelings can lead to binge eating as a coping mechanism. It focuses on mindfulness, emotion regulation, and distress tolerance. It is delivered in individual sessions or as group therapy. |
| Interpersonal psychotherapy |
| Psychotherapy that helps participants understand how problems with social interactions can lead to binge eating as a coping mechanism. It helps participants learn to cope with negative emotions stemming from problems with social interactions and develop healthy interpersonal skills. It is delivered in individual sessions or as group therapy. |
This Agency for Healthcare Research and Quality (AHRQ) review included 57 studies and one systematic review on the effectiveness and adverse effects of treatments for adults with binge-eating disorder. Meta-analyses provided strong evidence that lisdexamfetamine and second-generation antidepressants increase binge-eating abstinence. Second-generation antidepressants also decrease binge-eating frequency, and eating obsessions and compulsions. The U.S. Food and Drug Administration warns about the potential for abuse and dependence with lisdexamfetamine use, as well as an increased risk of sudden death, stroke, and myocardial infarction.2
Meta-analyses provided strong evidence that therapist-led CBT reduces binge-eating frequency and increases binge-eating abstinence. There is moderate evidence that behavioral weight loss therapy, which incorporates strategies such as caloric restriction and increased physical activity to promote weight loss, decreases body mass index more than CBT, but it is not clearly associated with reduced binge-eating behaviors. There was insufficient evidence to determine the effectiveness of other psychotherapies or combinations of pharmacologic and psychological treatments.
The AHRQ review is consistent with the American Psychiatric Association practice guideline for the treatment of patients with eating disorders, which strongly recommends individual and group CBT for binge-eating disorders, as well as guided self-help programs.5 There is little evidence regarding the optimal duration of treatment. One study suggested that 16 sessions of CBT are more effective than eight sessions.6 Another controlled study found that a 10-month sequence of CBT followed by behavioral weight loss therapy is not significantly superior to six-month courses of CBT or behaviorial weight loss therapy alone.7
In 2011, the World Federation of Societies of Biological Psychiatry Task Force on Eating Disorders identified 26 randomized controlled trials of pharmacologic treatments for binge-eating disorder and concluded that evidence supports the use of imipramine, sertraline, citalopram or escitalopram, and topiramate.8 The optimal treatment duration is unclear.
Based on the AHRQ review, a reasonable approach is to refer patients with binge-eating disorder to a therapist-led CBT program as first-line therapy. Patients who are unable to access a program or who do not respond to CBT may be prescribed lisdexamfetamine or a second-generation antidepressant. To find therapists who specialize in eating disorders, go to the http://locator.apa.org/.
editor's note: American Family Physician SOR ratings are different from the AHRQ Strength of Evidence (SOE) ratings.