
Am Fam Physician. 2017;95(6):366-372
Related letter: Group Prenatal Care to Reduce Preterm Labor and Improve Outcomes
Author disclosure: No relevant financial affiliations.
In the United States, preterm delivery is the leading cause of neonatal morbidity and is the most common reason for hospitalization during pregnancy. The rate of preterm delivery (before 37 weeks' gestation) has been declining since 2007. Clinical diagnosis of preterm labor is made if there are regular contractions and concomitant cervical change. Less than 10% of women with a clinical diagnosis of preterm labor will deliver within seven days of initial presentation. Women with a history of spontaneous preterm delivery are 1.5 to two times more likely to have a subsequent preterm delivery. Antenatal progesterone is associated with a significant decrease in subsequent preterm delivery in certain pregnant women. Current recommendations are to prescribe vaginal progesterone in women with a shortened cervix and no history of preterm delivery, and to use progesterone supplementation regardless of cervical length in women with a history of spontaneous preterm delivery. Cervical cerclage has been used to help correct structural defects or cervical weakening in high-risk women with a shortened cervix. A course of corticosteroids is the only antenatal intervention that has been shown to improve postdelivery neonatal outcomes, including a reduction in neonatal mortality, intracranial hemorrhage, necrotizing enterocolitis, and neonatal infection. Tocolytics, especially prostaglandin inhibitors and calcium channel blockers, may allow time for the administration of antenatal corticosteroids and transfer to a tertiary care facility if necessary. When used in specific at-risk populations, magnesium sulfate provides neuroprotection and decreases the incidence of cerebral palsy in preterm infants.
Spontaneous preterm delivery is the leading cause of neonatal morbidity in the United States and is the most common reason for hospitalization during pregnancy. The rate of all-cause preterm deliveries in the United States decreased from 10.4% in 2007 to 9.6% in 2015. In 2014, preterm deliveries occurred in 7.7% of single gestation pregnancies.1 Multiple gestation pregnancies are associated with increased rates of preterm delivery (50% of twins and 90% of triplets are delivered before 37 weeks' gestation).2 Preterm delivery (birth before 37 weeks' gestation) is further delineated into very early preterm (before 32 weeks), early preterm (32 0/7 to 33 6/7 weeks), and late preterm (34 0/7 to 36 6/7 weeks).
| Clinical recommendation | Evidence rating | References |
|---|---|---|
| In women with a single gestation pregnancy and a history of spontaneous preterm delivery, progesterone supplementation is beneficial starting at 16 to 24 weeks' gestation and continuing through 34 weeks' gestation. | A | 3, 12, 23–25 |
| Once preterm labor is confirmed, a single course of corticosteroids (betamethasone or dexamethasone) is the only intervention for improving neonatal outcomes. It is recommended between 24 and 34 weeks' gestation and may be considered as early as 23 weeks' gestation. | A | 21, 37 |
| Antenatal magnesium sulfate provides neuroprotection, decreasing the risk of cerebral palsy in infants born at less than 32 weeks' gestation. | B | 38–41 |
| Tocolytics, such as prostaglandin inhibitors and calcium channel blockers, should be used to prolong the time to delivery so that antenatal corticosteroids and potentially magnesium sulfate can be administered, and the mother can be transferred to a tertiary facility with a neonatal intensive care unit. | A | 21, 41 |
A recent reduction in preterm deliveries in the United States may partially be the result of newer recommendations from the American College of Obstetricians and Gynecologists. These recommendations include progesterone treatment for those at high risk of preterm delivery, stricter guidelines for assisted reproductive technology, and the reduction of elective early term deliveries before 39 weeks' gestation (because of the possibility of incorrect estimation of gestational age, these infants could potentially be born preterm).3–5 Premature infants have increased rates of morbidity and mortality in early and late childhood and decreased rates of unassisted pregnancy later in life, compared with term infants.6
Risk Factors for Preterm Delivery
There have been attempts to design a stratification tool to determine the risk of preterm delivery based on risk factors (Table 17 ). For instance, the rate of preterm delivery differs greatly between black and white women, with 2015 preliminary data showing a rate of 13.4% in blacks and 8.9% in non-Hispanic whites.2 Although many risk scoring systems have been developed, their ability to identify at-risk women and subsequently prevent a preterm delivery has not been evaluated.8 In the absence of such evidence, risk recognition and subsequent management of contributing factors are the best strategy to prevent preterm delivery.
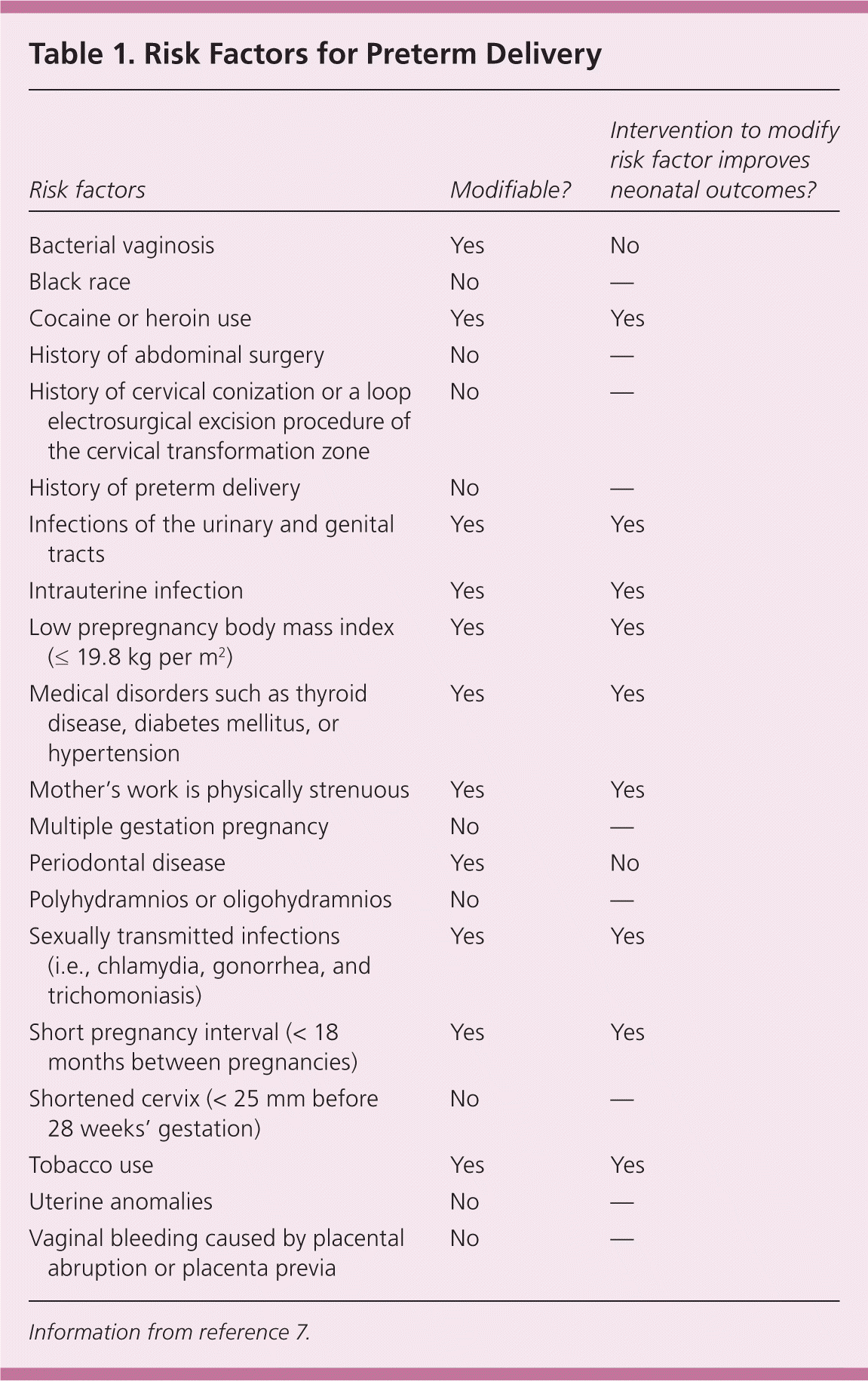
| Risk factors | Modifiable? | Intervention to modify risk factor improves neonatal outcomes? |
|---|---|---|
| Bacterial vaginosis | Yes | No |
| Black race | No | — |
| Cocaine or heroin use | Yes | Yes |
| History of abdominal surgery | No | — |
| History of cervical conization or a loop electrosurgical excision procedure of the cervical transformation zone | No | — |
| History of preterm delivery | No | — |
| Infections of the urinary and genital tracts | Yes | Yes |
| Intrauterine infection | Yes | Yes |
| Low prepregnancy body mass index (≤ 19.8 kg per m2) | Yes | Yes |
| Medical disorders such as thyroid disease, diabetes mellitus, or hypertension | Yes | Yes |
| Mother's work is physically strenuous | Yes | Yes |
| Multiple gestation pregnancy | No | — |
| Periodontal disease | Yes | No |
| Polyhydramnios or oligohydramnios | No | — |
| Sexually transmitted infections (i.e., chlamydia, gonorrhea, and trichomoniasis) | Yes | Yes |
| Short pregnancy interval (< 18 months between pregnancies) | Yes | Yes |
| Shortened cervix (< 25 mm before 28 weeks' gestation) | No | — |
| Tobacco use | Yes | Yes |
| Uterine anomalies | No | — |
| Vaginal bleeding caused by placental abruption or placenta previa | No | — |
Unmodifiable risk factors include a shortened cervix (less than 25 mm before 28 weeks' gestation) and a history of preterm delivery. The relative risk [RR] is 6.19 for a cervical length of 26 mm or less.9 Women with a prior preterm delivery have a 1.5- to twofold increased risk of a subsequent preterm delivery.9–12 Behavioral risk factors include low maternal prepregnancy body mass index (19.8 kg per m2 or less), maternal smoking (RR = 1.2 to 1.6 13), substance abuse, and a short pregnancy interval (less than 18 months between pregnancies).7,14,15
A history of cervical conization or a loop electrosurgical excision procedure of the cervical transformation zone also increases the risk of preterm delivery (RR = 1.99; 95% confidence interval [CI], 1.81 to 2.20).16,17 Infections of the urinary and genital tracts and periodontal disease have been associated with an increase in preterm delivery.18 However, treatment of these infections has not been shown to affect preterm delivery rates.3 Treatment of periodontal disease appeared to increase the risk of preterm delivery in one study.3,18,19
Other preventive strategies that have not shown benefit include vitamin C, vitamin E, bed rest, hydration, and home contraction monitoring.3,20,21 High fish consumption has been linked to a decreased incidence of preterm delivery, but taking omega-3 fatty acid supplements has not been shown to be beneficial.22
Strategies to Prevent Preterm Delivery
PROGESTERONE THERAPY
In women with single gestation pregnancy and a history of spontaneous preterm delivery, antenatal progesterone therapy is the most effective strategy to decrease the risk of a recurrent preterm delivery. Progesterone supplementation is beneficial in these women starting at 16 to 24 weeks' gestation and continuing through 34 weeks' gestation.3,12,23–25 The U.S. Food and Drug Administration has approved hydroxyprogesterone caproate (Makena), 250 mg intramuscularly, as weekly injections.23,26 Vaginal progesterone can be used in women with no history of spontaneous preterm delivery if they have a cervical length of 20 mm or less before 24 weeks' gestation. In a randomized placebo-controlled trial, treatment with vaginal micronized progesterone, 200 mcg daily, was associated with a 44% reduction in spontaneous preterm delivery in asymptomatic women with a cervical length of 15 mm or less at 20 to 25 weeks' gestation (RR = 0.56; 95% CI, 0.36 to 0.86).24 Progesterone is not beneficial in multiple gestation pregnancies.
CERVICAL CERCLAGE AND PESSARY
Cervical cerclage, an encircling suture placed around the cervix before or during pregnancy, has been used to help correct structural defects or cervical weakening in high-risk women with a shortened cervix. Studies have shown that cerclage is associated with a decrease in preterm delivery and in perinatal death when used in women with a prior preterm delivery and a cervical length of 25 mm or less.27 Although there are no randomized trials comparing cerclage with progesterone, a meta-analysis of several studies that evaluated the methods separately suggests that both treatments are beneficial. There is no evidence that they are more effective when used together.28 The benefit of cerclage in women with a short cervix but no history of a preterm delivery is uncertain. Cerclage is not recommended for multiple gestation pregnancies and has been associated with a twofold increased risk of preterm delivery (RR = 2.2; 95% CI, 1.2 to 4) in twin pregnancies.3
One trial evaluated the use of cervical pessary and found that the rate of delivery before 34 weeks' gestation was significantly lower in women with a pessary vs. those without a pessary (6% vs. 27%; odds ratio = 0.18; 95% CI, 0.08 to 0.37).13 With further studies, the cervical pessary may be a promising noninvasive additional intervention to prevent preterm delivery in women with a shortened cervix.3
Cervical Length Screening
The RR of preterm delivery increases with decreasing cervical length.9 Universal cervical length screening is controversial. There is concern about its cost-effectiveness, the availability of quality imaging for all patients, and the possibility of unnecessary interventions. If screening is performed, cervical length should be measured transvaginally by a qualified ultrasonographer.
Evaluation of Patients in Preterm Labor
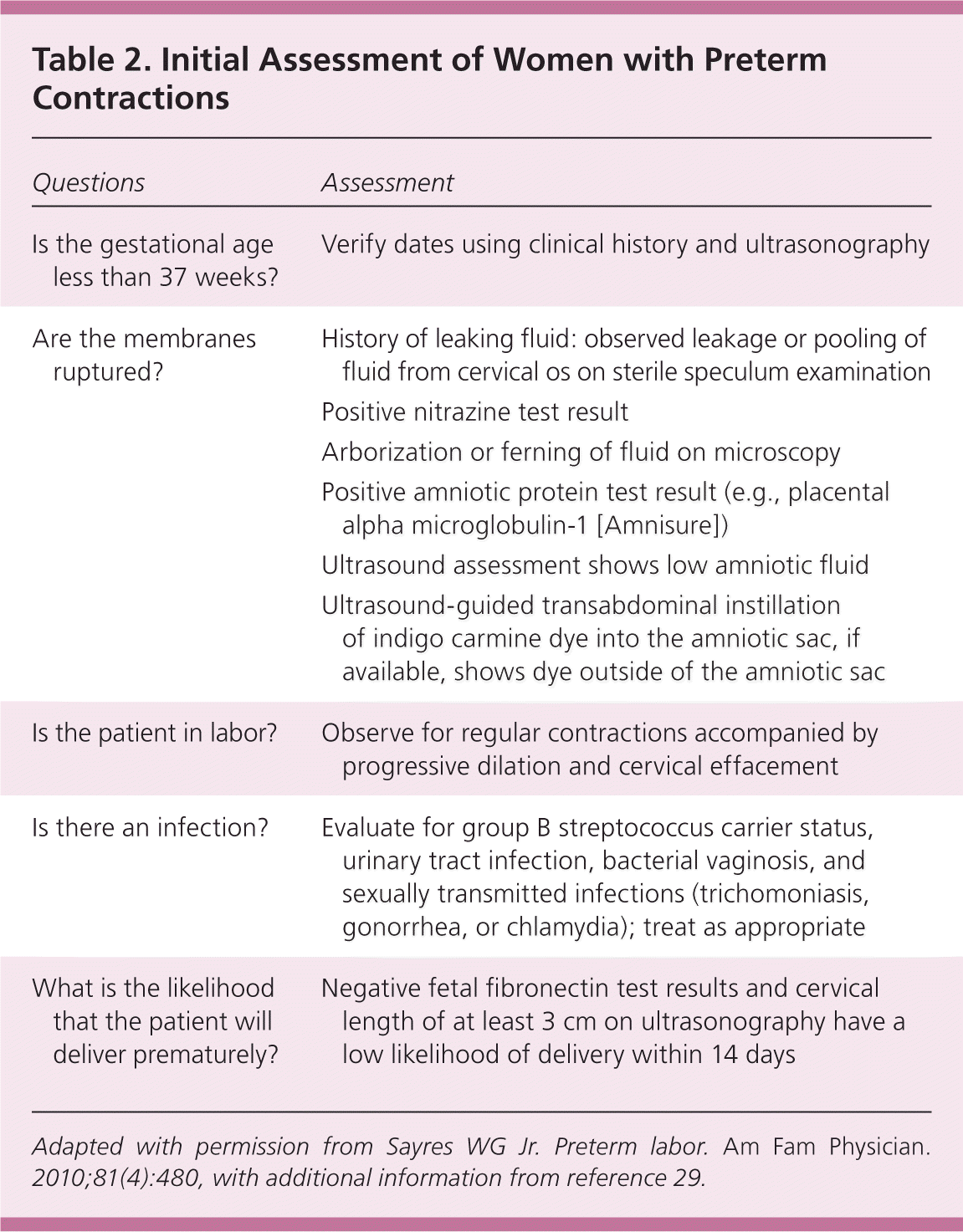
Less than 10% of women with a clinical diagnosis of preterm labor will deliver within seven days of initial presentation.21 It is therefore essential to determine if the patient is in true labor or if delivery is imminent. An algorithm for preterm labor assessment is available at http://www.marchofdimes.org/pdf/nevada/nv-Preterm-Labor-Assessment-Toolkit.pdf.
When a patient has fluid loss, contractions, pelvic pressure, or abdominal pain, a complete medical and obstetric history should be obtained to assess the patient's risks, including verification of the estimated due date. Unless delivery seems imminent, a digital vaginal examination should be avoided because it could increase the risk of infection. A sterile speculum examination should be performed for further risk stratification, including evaluation for cervical dilation, vaginal infections, bleeding, and rupture of membranes. Because infections have been associated with preterm delivery, patients should be tested for sexually transmitted infections and group B streptococcal infection. A urine sample should be collected to check for urinary tract infection.29
A positive nitrazine test result (pH of 7.1 to 7.3), arborization or ferning on microscopy, and pooling of fluid in the vaginal vault during speculum examination are indicators of ruptured membranes. Tests for amniotic proteins, such as placental alpha microglobulin-1 (Amnisure), have high reported sensitivity for premature rupture of membranes.29 Other methods for confirming premature rupture of membranes include ultrasound assessment of low amniotic fluid levels and ultrasound-guided transabdominal instillation of indigo carmine dye into the amniotic sac. Most women presenting with preterm premature rupture of membranes will deliver within one week.7 Once preterm premature rupture of membranes has been confirmed, management depends on the status of the fetus and the presence of placental abruption or intrauterine infection. Fetal compromise, clinical chorioamnionitis, and significant abruptio placentae are clear indications for delivery.29
Preterm labor is diagnosed when a patient is having regular uterine contractions that are accompanied by progressive dilation and cervical effacement. Accuracy of the clinical diagnosis is increased when there are at least six contractions per hour, cervical dilation is more than 3 cm, effacement is at least 80%, membranes are ruptured, or there is vaginal bleeding.30
| Questions | Assessment |
|---|---|
| Is the gestational age less than 37 weeks? | Verify dates using clinical history and ultrasonography |
| Are the membranes ruptured? | History of leaking fluid: observed leakage or pooling of fluid from cervical os on sterile speculum examination |
| Positive nitrazine test result | |
| Arborization or ferning of fluid on microscopy | |
| Positive amniotic protein test result (e.g., placental alpha microglobulin-1 [Amnisure]) | |
| Ultrasound assessment shows low amniotic fluid | |
| Ultrasound-guided transabdominal instillation of indigo carmine dye into the amniotic sac, if available, shows dye outside of the amniotic sac | |
| Is the patient in labor? | Observe for regular contractions accompanied by progressive dilation and cervical effacement |
| Is there an infection? | Evaluate for group B streptococcus carrier status, urinary tract infection, bacterial vaginosis, and sexually transmitted infections (trichomoniasis, gonorrhea, or chlamydia); treat as appropriate |
| What is the likelihood that the patient will deliver prematurely? | Negative fetal fibronectin test results and cervical length of at least 3 cm on ultrasonography have a low likelihood of delivery within 14 days |
When further evaluation is necessary to predict the likelihood of a preterm delivery, fetal fibronectin testing and cervical length ultrasonography may be helpful. Fetal fibronectin is a glycoprotein produced by amniocytes and cytotrophoblasts. It appears in cervical secretions before the onset of labor. The fetal fibronectin test has a high negative predictive value. A patient who tests negative has a low probability of delivery within the next 14 days.30,32–34 However, collection of fetal fibronectin is not recommended within 48 hours of sexual intercourse or in the presence of moderate vaginal bleeding because these can falsely elevate fetal fibronectin concentrations.34 The use of a quantitative fetal fibronectin test shows promise for even more precise prediction of delivery within 14 days.34 When performed correctly, transvaginal cervical length measurement of at least 3 cm is associated with a low likelihood of delivery within 14 days.30,35 Further, it appears there is additive benefit when fetal fibronectin testing and cervical length measurement are used together.30,32–36
Management of Preterm Labor
CORTICOSTEROID THERAPY
Once preterm labor is confirmed, a single course of corticosteroids is the only intervention for improving neonatal outcomes. Betamethasone, two 12-mg doses given intramuscularly 24 hours apart, or dexamethasone, four 6-mg doses given intramuscularly every 12 hours, is recommended between 24 and 34 weeks' gestation, and may be considered as early as 23 weeks' gestation, in women likely to deliver within seven days regardless of membrane status.21,37
The use of corticosteroids is associated with decreased neonatal morbidity and mortality.21 Infants whose mothers receive antenatal corticosteroids are less likely to exhibit respiratory distress syndrome, intraventricular hemorrhage, and necrotizing enterocolitis compared with those whose mothers did not receive corticosteroids.21 Recent data suggest that a second rescue course of antenatal corticosteroids may be considered if the first dose was given more than seven days earlier and there is still a risk of preterm delivery before 34 weeks' gestation.21
MAGNESIUM SULFATE
Because of its neuroprotective effect, administration of antenatal magnesium sulfate has been associated with a decrease in occurrence and severity of cerebral palsy in infants.38–40 A 2009 Cochrane review revealed that antenatal magnesium sulfate therapy in women at risk of preterm delivery substantially reduced the risk of cerebral palsy in their infants (RR = 0.69; 95% CI, 0.54 to 0.87).38 Because magnesium sulfate can cause maternal complications (e.g., respiratory depression, cardiac arrest), following institutional protocols is suggested for determining appropriate use.21,38–40
TOCOLYSIS
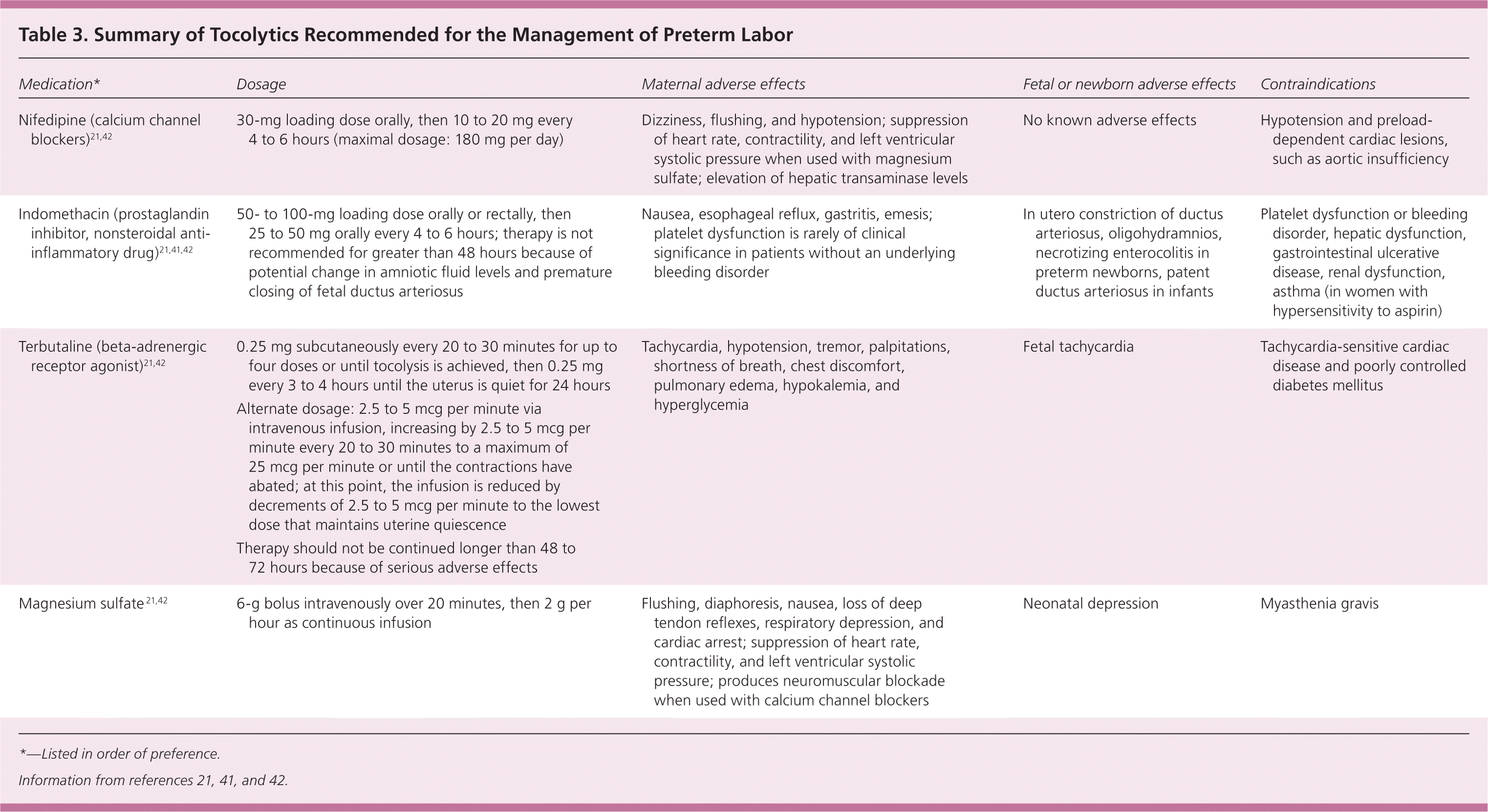
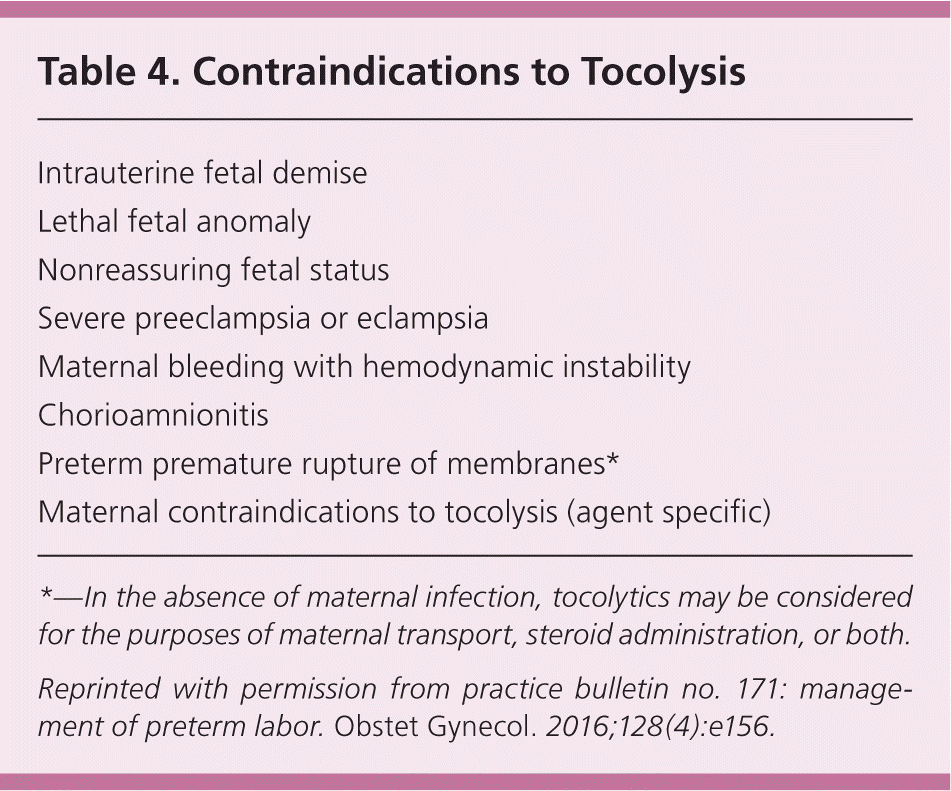
The role of tocolytic agents (Table 3)21,41,42 is to prolong the time to delivery so that antenatal corticosteroids and potentially magnesium sulfate can be administered, and the mother can be transferred to a tertiary care facility with a neonatal intensive care unit. Tocolytics have not been shown to directly improve neonatal outcomes,21 and they are not always indicated.
| Medication* | Dosage | Maternal adverse effects | Fetal or newborn adverse effects | Contraindications |
|---|---|---|---|---|
| Nifedipine (calcium channel blockers)21,42 | 30-mg loading dose orally, then 10 to 20 mg every 4 to 6 hours (maximal dosage: 180 mg per day) | Dizziness, flushing, and hypotension; suppression of heart rate, contractility, and left ventricular systolic pressure when used with magnesium sulfate; elevation of hepatic transaminase levels | No known adverse effects | Hypotension and preload-dependent cardiac lesions, such as aortic insufficiency |
| Indomethacin (prostaglandin inhibitor, nonsteroidal anti-inflammatory drug)21,41,42 | 50- to 100-mg loading dose orally or rectally, then 25 to 50 mg orally every 4 to 6 hours; therapy is not recommended for greater than 48 hours because of potential change in amniotic fluid levels and premature closing of fetal ductus arteriosus | Nausea, esophageal reflux, gastritis, emesis; platelet dysfunction is rarely of clinical significance in patients without an underlying bleeding disorder | In utero constriction of ductus arteriosus, oligohydramnios, necrotizing enterocolitis in preterm newborns, patent ductus arteriosus in infants | Platelet dysfunction or bleeding disorder, hepatic dysfunction, gastrointestinal ulcerative disease, renal dysfunction, asthma (in women with hypersensitivity to aspirin) |
| Terbutaline (beta-adrenergic receptor agonist)21,42 |
| Tachycardia, hypotension, tremor, palpitations, shortness of breath, chest discomfort, pulmonary edema, hypokalemia, and hyperglycemia | Fetal tachycardia | Tachycardia-sensitive cardiac disease and poorly controlled diabetes mellitus |
| Magnesium sulfate 21,42 | 6-g bolus intravenously over 20 minutes, then 2 g per hour as continuous infusion | Flushing, diaphoresis, nausea, loss of deep tendon reflexes, respiratory depression, and cardiac arrest; suppression of heart rate, contractility, and left ventricular systolic pressure; produces neuromuscular blockade when used with calcium channel blockers | Neonatal depression | Myasthenia gravis |
First-line agents used to delay delivery up to 48 hours include calcium channel blockers (e.g., nifedipine, nicardipine), beta-adrenergic receptor agonists (e.g., terbutaline), and nonsteroidal anti-inflammatory drugs such as prostaglandin inhibitors (e.g., indomethacin, ketorolac).41 A systematic review and network meta-analysis show that prostaglandin inhibitors and calcium channel blockers are the best tocolytics based on four outcomes: delay of delivery by 48 hours, neonatal mortality, neonatal respiratory distress syndrome, and maternal adverse effects (all causes).43 Magnesium sulfate can be used as a tocolytic but is associated with significant maternal adverse effects. Caution must be used if combining magnesium sulfate with beta-adrenergic receptor agonists or calcium channel blockers because of possible maternal complications. Prostaglandin inhibitors may be used in combination with magnesium sulfate with lower maternal adverse effects; however, use of prostaglandin inhibitors after 32 weeks' gestation may be associated with premature closure of the ductus arteriosus in the infant.21
| Intrauterine fetal demise |
| Lethal fetal anomaly |
| Nonreassuring fetal status |
| Severe preeclampsia or eclampsia |
| Maternal bleeding with hemodynamic instability |
| Chorioamnionitis |
| Preterm premature rupture of membranes* |
| Maternal contraindications to tocolysis (agent specific) |
ANTIBIOTICS
Intrauterine bacterial infections are associated with preterm labor, especially before 32 weeks' gestation. Although several trials have been conducted, no studies have shown that use of antibiotics during preterm labor is effective in delaying delivery or reducing neonatal morbidity associated with preterm delivery.21 This lack of benefit has no bearing on established guidelines recommending the use of antibiotics for group B streptococcus prophylaxis and in women with premature rupture of membranes.
This article updates previous articles on this topic by Sayres31 ; Medina and Hill44 ; Weismiller45 ; and Von Der Pool.46
Data Sources: The following databases were searched: Essential Evidence Plus, PubMed, Cochrane Database of Systematic Reviews, U.S. Preventive Services Task Force, and ACOG practice bulletins. Search dates: March 30 to April 1, 2016; July 21, 2016; and November 15 to 18, 2016.
