Am Fam Physician. 2017;95(10):online
As published by the U.S. Preventive Services Task Force.
Summary of Recommendation and Evidence
The USPSTF recommends that all women who are planning or capable of pregnancy take a daily supplement containing 0.4 to 0.8 mg (400 to 800 μg) of folic acid (Table 1). A recommendation.
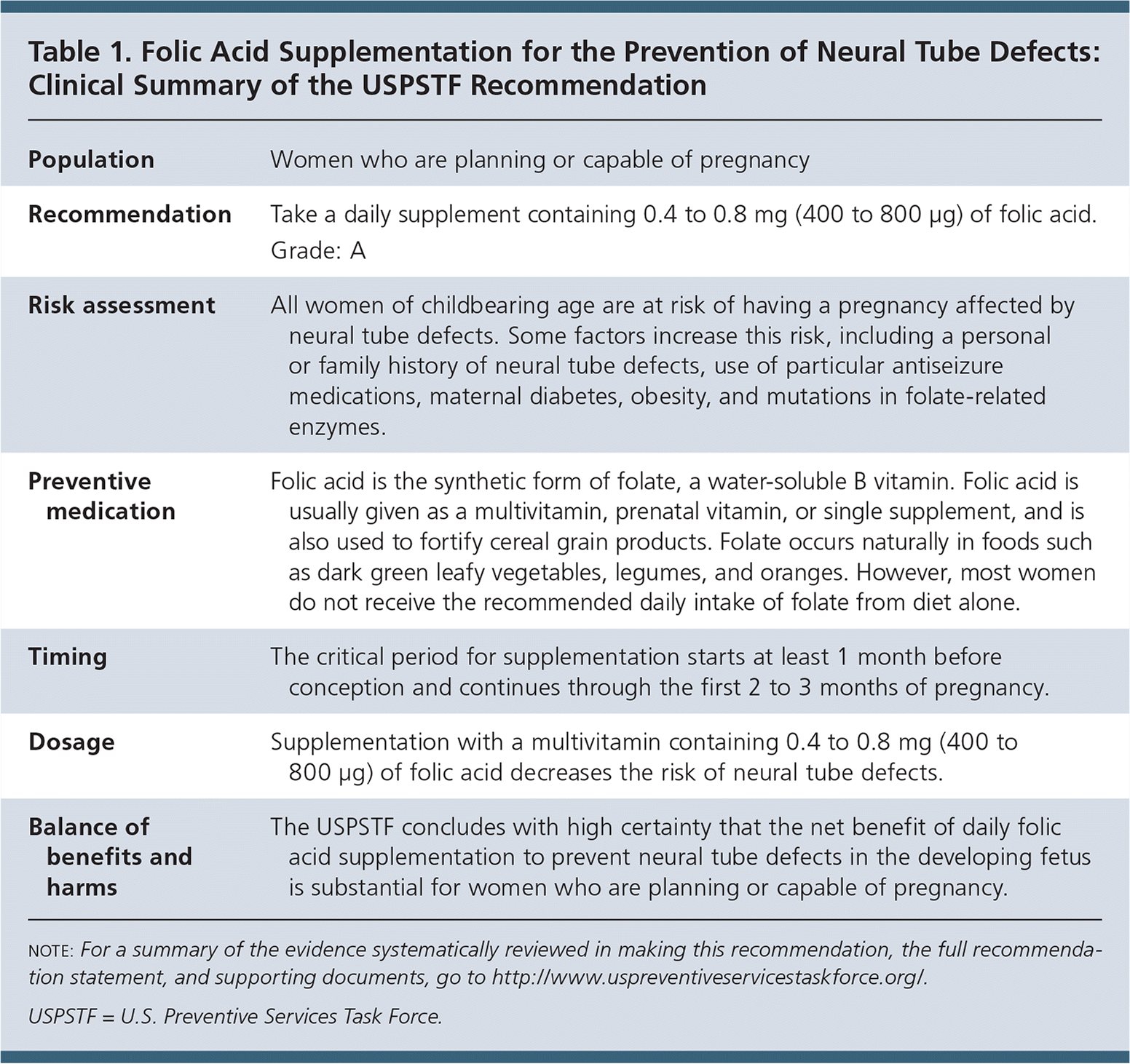
| Population | Women who are planning or capable of pregnancy |
| Recommendation | Take a daily supplement containing 0.4 to 0.8 mg (400 to 800 μg) of folic acid. Grade: A |
| Risk assessment | All women of childbearing age are at risk of having a pregnancy affected by neural tube defects. Some factors increase this risk, including a personal or family history of neural tube defects, use of particular antiseizure medications, maternal diabetes, obesity, and mutations in folate-related enzymes. |
| Preventive medication | Folic acid is the synthetic form of folate, a water-soluble B vitamin. Folic acid is usually given as a multivitamin, prenatal vitamin, or single supplement, and is also used to fortify cereal grain products. Folate occurs naturally in foods such as dark green leafy vegetables, legumes, and oranges. However, most women do not receive the recommended daily intake of folate from diet alone. |
| Timing | The critical period for supplementation starts at least 1 month before conception and continues through the first 2 to 3 months of pregnancy. |
| Dosage | Supplementation with a multivitamin containing 0.4 to 0.8 mg (400 to 800 μg) of folic acid decreases the risk of neural tube defects. |
| Balance of benefits and harms | The USPSTF concludes with high certainty that the net benefit of daily folic acid supplementation to prevent neural tube defects in the developing fetus is substantial for women who are planning or capable of pregnancy. |
Rationale
IMPORTANCE
Neural tube defects are major birth defects of the brain and spine that occur early in pregnancy due to improper closure of the embryonic neural tube, which may lead to a range of disabilities or death. The most common neural tube defects are anencephaly (an underdeveloped brain and an incomplete skull) and spina bifida (incomplete closing of the spinal cord).1,2 Based on 2009–2011 data, the estimated average annual prevalence of anencephaly and spina bifida combined was 6.5 cases per 10,000 live births.1–3 Daily folic acid supplementation in the periconceptional period can prevent neural tube defects.1,2
Folic acid is the synthetic form of folate, a water-soluble B vitamin (B9). Folic acid is usually given as a multivitamin, prenatal vitamin, or single supplement. It is also used to fortify cereal grain products. Folate occurs naturally in foods such as dark green leafy vegetables, legumes, and oranges.1 However, most women do not receive the recommended daily intake of folate from diet alone.1 National Health and Nutrition Examination Survey (NHANES) data from 2003 to 2006 suggest that 75% of nonpregnant women aged 15 to 44 years do not consume the recommended daily intake of folic acid for preventing neural tube defects.1,2,4
RECOGNITION OF RISK STATUS
Women who have a personal or family history of a pregnancy affected by a neural tube defect are at increased risk of having an affected pregnancy. However, most cases occur in the absence of any personal or family history.
BENEFITS OF PREVENTIVE MEDICATION
The USPSTF found convincing evidence that folic acid supplementation in the periconceptional period provides substantial benefits in reducing the risk of neural tube defects in the developing fetus. The USPSTF found inadequate evidence on how the benefits of folic acid supplementation may vary by dosage, timing relative to pregnancy, duration of therapy, or race/ethnicity.
HARMS OF PREVENTIVE MEDICATION
The USPSTF found adequate evidence that the harms to the mother or infant from folic acid supplementation taken at the usual doses are no greater than small.
USPSTF ASSESSMENT
The USPSTF concludes with high certainty that the net benefit of daily folic acid supplementation to prevent neural tube defects in the developing fetus is substantial for women who are planning or capable of pregnancy.
Clinical Considerations
PATIENT POPULATION UNDER CONSIDERATION
This recommendation applies to women who are planning or capable of pregnancy. It does not apply to women who have had a previous pregnancy affected by neural tube defects or who are at very high risk due to other factors (e.g., use of certain antiseizure medications or family history). These women may be advised to take higher doses of folic acid.
ASSESSMENT OF RISK
Although all women of childbearing age are at risk of having a pregnancy affected by neural tube defects and should take folic acid supplementation, some factors increase their risk, including a personal or family history (first- or second-degree relative) of neural tube defects.1 Women with a personal history of an affected pregnancy require special care and are not within the scope of this recommendation statement. Other risk factors include the use of particular antiseizure medications (e.g., valproic acid or carbamazepine), maternal diabetes, obesity, and mutations in folate-related enzymes.1
Questions persist regarding increased risk of neural tube defects in some racial/ethnic groups. Birth prevalence rates are highest among Hispanic women, followed by non-Hispanic white and non-Hispanic black women.1 Genetic mutations in folate-related enzymes may vary by race/ethnicity. Dietary folate or folic acid intake differs by race/ethnicity. For example, Mexican American women may be at increased risk because of decreased consumption of fortified foods and greater intake of corn masa–based diets.1 Fewer Hispanic women (28%) report consuming 0.4 mg (400 μg) or more of folic acid daily through fortified food or supplements, compared with 39% of non-Hispanic white women.1,5
TIMING
Half of all pregnancies in the United States are unplanned.6 Therefore, clinicians should advise all women who are capable of pregnancy to take daily folic acid supplements. The critical period for supplementation starts at least 1 month before conception and continues through the first 2 to 3 months of pregnancy.1,7,8
DOSAGE
Trials and observational studies conducted in settings without food fortification suggest that supplementation with a multivitamin containing 0.4 to 0.8 mg (400 to 800 μg) of folic acid decreases the risk of neural tube defects.1,7,8 Evidence shows that most women in the United States are not consuming fortified foods in a quantity needed to demonstrate optimal benefit.8 An analysis of NHANES data found that 48% of respondents of childbearing age consumed the recommended amount of folic acid from mandatorily fortified foods only.1,9
According to the National Academy of Sciences Food and Nutrition Board, the tolerable upper intake level of folic acid in women 19 years and older is 1 mg/d (1,000 μg/d) from supplements or fortified food (excluding naturally occurring folate) and 0.8 mg/d (800 μg/d) for those aged 14 to 18 years.10 Fewer than 3% of girls and women aged 14 to 50 years receive more than 1 mg/d (1,000 μg/d) of folic acid from supplements or food.3,11,12
ADDITIONAL APPROACHES TO PREVENTION
The Community Preventive Services Task Force recommends community-wide education campaigns to encourage women of child-bearing age to take folic acid supplements.13
In 2016, the U.S. Food and Drug Administration approved folic acid fortification of corn masa flour. This allows manufacturers to voluntarily add folic acid to corn masa flour at levels consistent with those found in other enriched cereal grains.14