Am Fam Physician. 2017;95(10):664-666
Author disclosure: No relevant financial affiliations.
Sofosbuvir/velpatasvir (Epclusa) is an oral medication labeled for the treatment of adults who have chronic infection with hepatitis C virus (HCV) genotypes 1 through 6. Unlike elbasvir/grazoprevir (Zepatier) and ledipasvir/sofosbuvir (Harvoni), sofosbuvir/velpatasvir can be used to treat patients with genotypes 2 and 3. It is also labeled for use in combination with ribavirin to treat patients with decompensated cirrhosis (Child-Pugh score of B or C).1,2
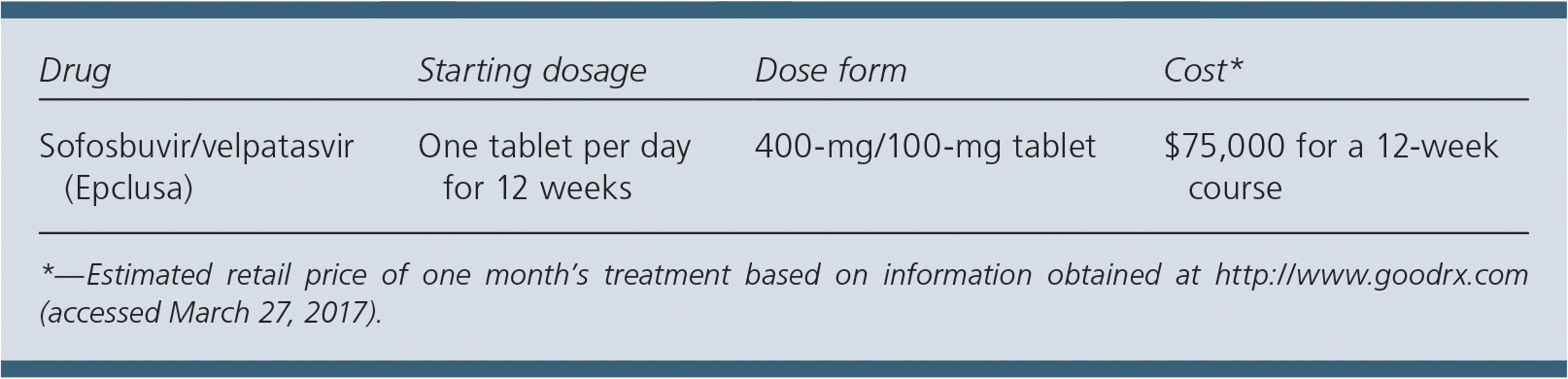
| Drug | Starting dosage | Dose form | Cost* |
|---|---|---|---|
| Sofosbuvir/velpatasvir (Epclusa) | One tablet per day for 12 weeks | 400-mg/100-mg tablet | $75,000 for a 12-week course |
SAFETY
The main safety concern with sofosbuvir/velpatasvir is reactivation of hepatitis B virus (HBV) in coinfected patients, which is unusual but may happen with any treatment of HCV infection. All patients should be tested for HBV before starting therapy by measuring hepatitis B surface antigen (HBsAg) and anti–hepatitis B core (anti-HBc) antibody. Patients with serologic evidence of HBV infection should be monitored for clinical and laboratory signs of hepatitis flare-up or HBV reactivation during treatment and posttreatment follow-up.1,2
Sofosbuvir/velpatasvir affects or is affected by numerous medications, and a suitable drug interaction reference should be consulted before beginning treatment or when considering additional medications during treatment.1,2 Liver enzyme inducers such as rifampin, St. John’s wort, and carbamazepine (Tegretol), along with others, will decrease the therapeutic effect of sofosbuvir/velpatasvir. Proton pump inhibitors should not be taken with sofosbuvir/velpatasvir; histamine H2 receptor blockers and antacids may be used, but there are specific timing guidelines in the product labeling. Interactions with antiarrhythmics (especially amiodarone) and anticonvulsants can cause significant adverse effects. Sofosbuvir/velpatasvir has not been studied in pregnant or breastfeeding women.1
TOLERABILITY
Sofosbuvir/velpatasvir is generally well tolerated, with only 0.2% of patients discontinuing treatment in clinical trials because of adverse effects. Fatigue and headaches are the most commonly reported adverse effects.1
EFFECTIVENESS
Among patients without cirrhosis or with compensated cirrhosis, 95% to 99% will achieve sustained virologic response (defined as HCV RNA less than 15 IU per mL at 12 weeks after completion of treatment; sustained virologic response is only a biomarker for cure of HCV infection and does not imply direct effects on morbidity or mortality). In one small study comparing sofosbuvir/velpatasvir (n = 64) with placebo (n = 116) in patients with HCV genotypes 1, 2, 4, 5, and 6, 99% of the patients taking sofosbuvir/velpatasvir achieved sustained virologic response vs. none of the patients treated with placebo.1,3 In patients with HCV genotype 3, sofosbuvir/velpatasvir is more effective than sofosbuvir/ribavirin, with response rates of 95% vs. 80%, respectively.1,4
In patients with decompensated cirrhosis (Child-Pugh score of B), 94% will achieve sustained virologic response when treated with sofosbuvir/velpatasvir in combination with ribavirin.1,5 There are no studies comparing the effectiveness of sofosbuvir/velpatasvir with elbasvir/grazoprevir or ledipasvir/sofosbuvir.
PRICE
A complete 12-week course of sofosbuvir/velpatasvir will cost approximately $75,000. This price is in the same range as elbasvir/grazoprevir ($60,000 for a 12-week course; $80,000 for a 16-week course) and ledipasvir/sofosbuvir ($94,000 for a 12-week course). Adding 12 weeks of ribavirin (1,000 mg per day) will cost approximately $550 to $850 more.2
SIMPLICITY
Sofosbuvir/velpatasvir is taken orally once daily with or without food. It does not require adjustment for renal or hepatic disease.
Bottom Line
Sofosbuvir/velpatasvir is an effective treatment for patients with HCV and has minimal adverse effects. It is the preferred treatment for patients with genotype 2 or 3. As with other curative treatments, it is very expensive. Patients should be instructed to avoid using proton pump inhibitors and only take antacids and H2 blockers if timed appropriately during treatment.