
Am Fam Physician. 2017;95(12):786-794
Related editorial: Strategies for Addressing and Overcoming Vaccine Hesitancy.
Patient information: See related handout on vaccinations, written by the authors of this article.
Author disclosure: No relevant financial affiliations.
Vaccines are one of the most successful medical advances in modern times. Most vaccine-preventable illnesses are unfamiliar to modern parents. Because of this, parents are increasingly questioning the necessity of immunizing their children, especially because no vaccine is completely free of adverse effects or the risk of complications. Family physicians should be aware of the risks and benefits of recommended immunizations. Thimerosal is currently used only in multidose vials of influenza vaccine, and exposure through vaccines is not associated with adverse neurologic outcomes. The measles, mumps, and rubella vaccine is not associated with autism. Vaccines are associated with local reactions, such as pain and erythema. The rotavirus vaccine minimally increases the rate of intussusception, whereas other vaccines minimally increase the risk of syncope. Although immunization with the human papillomavirus vaccine is recommended for all boys and girls, vaccination rates remain low. Physicians should guide parents to credible resources if they are considering vaccine refusal. If a recommended vaccine is refused, proper documentation is essential. The Vaccine Adverse Event Reporting System and National Vaccine Injury Compensation Program track adverse events and allow compensation for documented harms from vaccinations.
With the success of vaccinations, many parents no longer have contact with children who have vaccine-preventable illnesses. Because of this, parents have difficulty balancing the potential harms and benefits of vaccines. Some parents express concern that physicians are not well educated on the adverse effects of vaccines or that physicians purposefully withhold information on adverse effects.1 Parents may seek out additional information from websites containing inaccurate information. Family physicians should gather accurate information about the harms and benefits of vaccines to advocate for vaccination and decrease the incidence of vaccine-preventable diseases.
WHAT IS NEW ON THIS TOPIC: VACCINE ADVERSE EVENTS
In 2012, the United States had its largest pertussis outbreak since 1955, including 48,277 cases and 20 pertussis-related deaths.
The most common adverse effects of the human papillomavirus vaccine are transient and similar to those of other vaccines, including mild pain and bruising at the injection site, headache, lightheadedness, and syncope.
The American Academy of Family Physicians does not support immunization exemption policies except in cases of allergic or medical contraindication.
| Clinical recommendation | Evidence rating | References |
|---|---|---|
| A 25-mm needle should be used instead of a 16-mm needle to reduce the risk of adverse reactions to vaccinations. | A | 3 |
| Antipyretics are not recommended for routine prophylaxis before immunizations. | A | 6, 7 |
| The measles, mumps, and rubella vaccine does not increase the risk of autism and should be routinely used. | C | 35, 36 |
| Parents who refuse a recommended vaccine should sign a refusal to vaccinate form. | C | 7, 79, 82 |
Vaccine Adverse Events
Common local reactions to vaccines include pain, swelling, and erythema at the injection site. Systemic reactions, including fever, irritability, drowsiness, and rash, may also occur.2 Use of a longer needle (25 mm vs. 16 mm) decreases injection site reactions.3 The fourth dose of the diphtheria and tetanus toxoids and acellular pertussis (DTaP) vaccine is associated with an increased incidence of fever and injection site reactions compared with the first dose (one in four children).2 One out of 30 children reports up to seven days of swelling of the entire thigh or upper arm after the fourth or fifth dose.2 Syncope may occur—especially in adolescents—after administration of the human papillomavirus (HPV) vaccine; quadrivalent meningococcal conjugate vaccine (MCV4); or tetanus toxoid, reduced diphtheria toxoid, and acellular pertussis (Tdap) vaccine.4 Because of this, adolescents should be observed for 15 minutes after receiving these vaccines.2,5 Administration of acetaminophen at the time of vaccination or shortly afterward may alleviate some adverse effects, but there may be a decreased antibody response to some vaccine antigens in children who receive antipyretics.6 For this reason and because antipyretics do not prevent febrile seizures, the Centers for Disease Control and Prevention (CDC) no longer recommends routine prophylaxis before vaccination.7
Potential Allergens in Vaccines
Trace amounts of antibiotics, such as neomycin, are present in varicella; measles, mumps, and rubella (MMR); and inactivated poliovirus vaccines. Therefore, these vaccines are contraindicated in patients with a history of anaphylactic reaction to these antibiotics.8,9 Gelatin is used in some live virus vaccines, such as varicella and MMR; these vaccines should not be given to patients with a history of anaphylaxis to gelatin.8 Egg allergy is not a contraindication to the MMR vaccine, even though it is derived from chick embryo fibroblast tissue culture.9 Patients with anaphylaxis to egg proteins should not receive the inactivated influenza vaccine and should receive the egg-free trivalent inactivated influenza vaccine instead.10 The CDC publishes tables detailing the inactive ingredients or excipients and other potential allergens found in vaccines.8,11 Anaphylactic reactions to vaccines occur in approximately one per 1 million doses.12 A patient who has an anaphylactic reaction to a vaccine should undergo immediate type allergy skin testing to confirm that the reaction was immunoglobulin E mediated and to determine which vaccine component was responsible. If the skin test is negative, the vaccine can be readministered under close observation with resuscitation, including epinephrine, readily available.10,12 Every effort should be made to avoid unnecessarily withholding a disease-preventing vaccine. Table 1 lists serious adverse reactions to vaccines.8,11–26
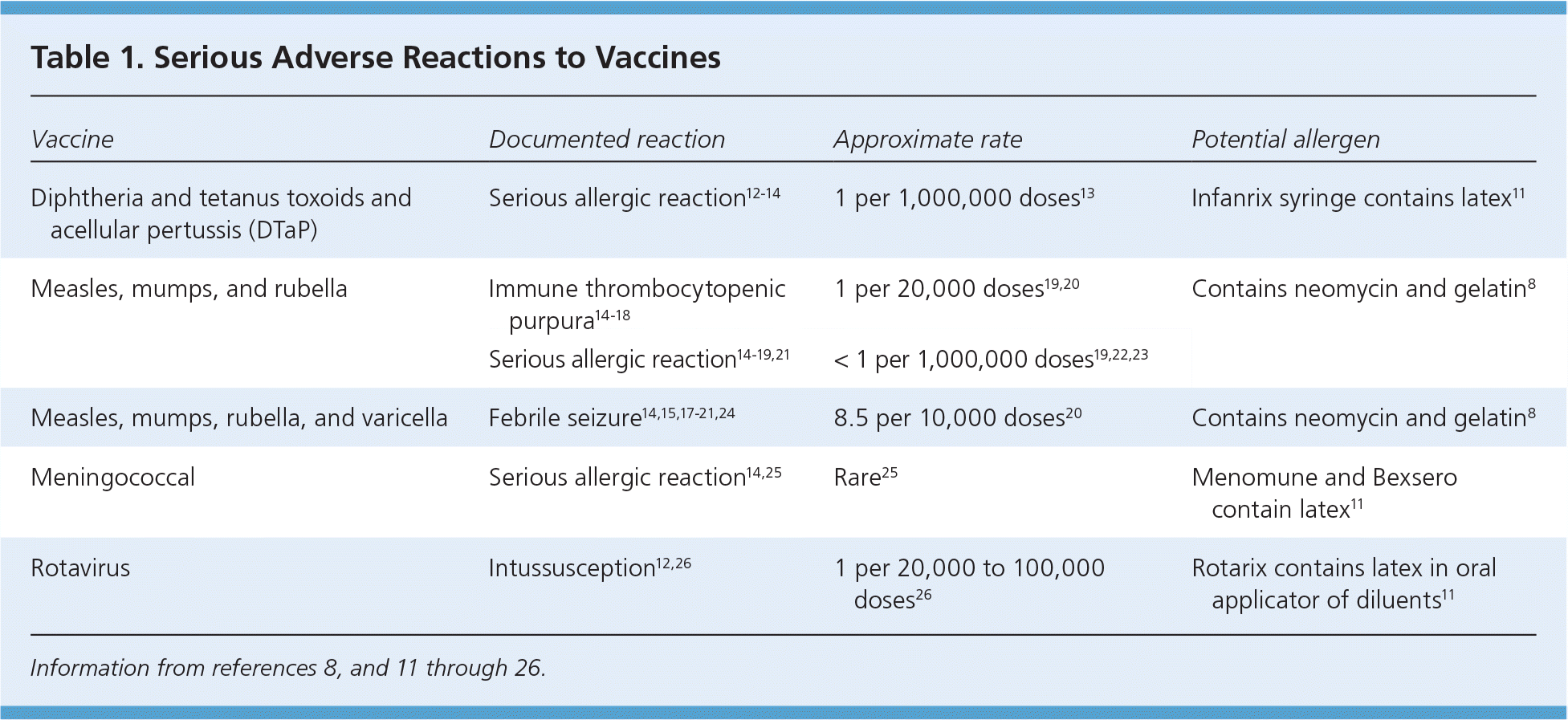
| Vaccine | Documented reaction | Approximate rate | Potential allergen |
|---|---|---|---|
| Diphtheria and tetanus toxoids and acellular pertussis (DTaP) | Serious allergic reaction12–14 | 1 per 1,000,000 doses13 | Infanrix syringe contains latex11 |
| Measles, mumps, and rubella | Immune thrombocytopenic purpura14–18 | 1 per 20,000 doses19,20 | Contains neomycin and gelatin8 |
| Serious allergic reaction14–19,21 | < 1 per 1,000,000 doses19,22,23 | ||
| Measles, mumps, rubella, and varicella | Febrile seizure14,15,17–21,24 | 8.5 per 10,000 doses20 | Contains neomycin and gelatin8 |
| Meningococcal | Serious allergic reaction14,25 | Rare25 | Menomune and Bexsero contain latex11 |
| Rotavirus | Intussusception12,26 | 1 per 20,000 to 100,000 doses26 | Rotarix contains latex in oral applicator of diluents11 |
Thimerosal in Vaccines
Thimerosal, a preservative containing ethyl mercury, was used to prevent bacterial and fungal contamination of vaccines beginning in the 1930s. In 1999, the U.S. Food and Drug Administration (FDA) determined that the child immunization schedule may expose infants to more mercury than recommended by the Environmental Protection Agency.27–29 There is no link between thimerosal exposure and adverse outcomes such as autism or abnormal neuropsychological functioning.30–32 Thimerosal has been removed from all routine childhood vaccines except multidose vials of influenza vaccines.33 Inactivated influenza vaccines are formulated in both multidose vials and preservative-free single-dose vials. Annual exposure to a thimerosal-containing influenza vaccine is considered safe.34
MMR Vaccine
An article linking autism to the MMR vaccine was retracted for fraud, but this misinformation persists and has caused long-lasting public health consequences.35,36 Multiple studies have found no causal link between vaccination and autism, but the falsified report continues to cause parental concern.35 Physicians must acknowledge these fears and inform concerned parents about the benefits of immunization and the potential harm of vaccine refusal. Febrile seizures that occur seven to 10 days after the first dose of the MMR vaccine are uncommon, but the risk is increased when the first dose is given after the recommended age of 12 to 15 months.37,38 The risk of immune thrombocytopenic purpura is increased after both natural measles infection and for up to six weeks after MMR vaccination (Table 2).16,18,19,21–24,39–51 Infection with measles, mumps, or rubella causes more severe adverse effects than the MMR vaccine, and vaccine refusal has been linked to recent outbreaks of these infections.45
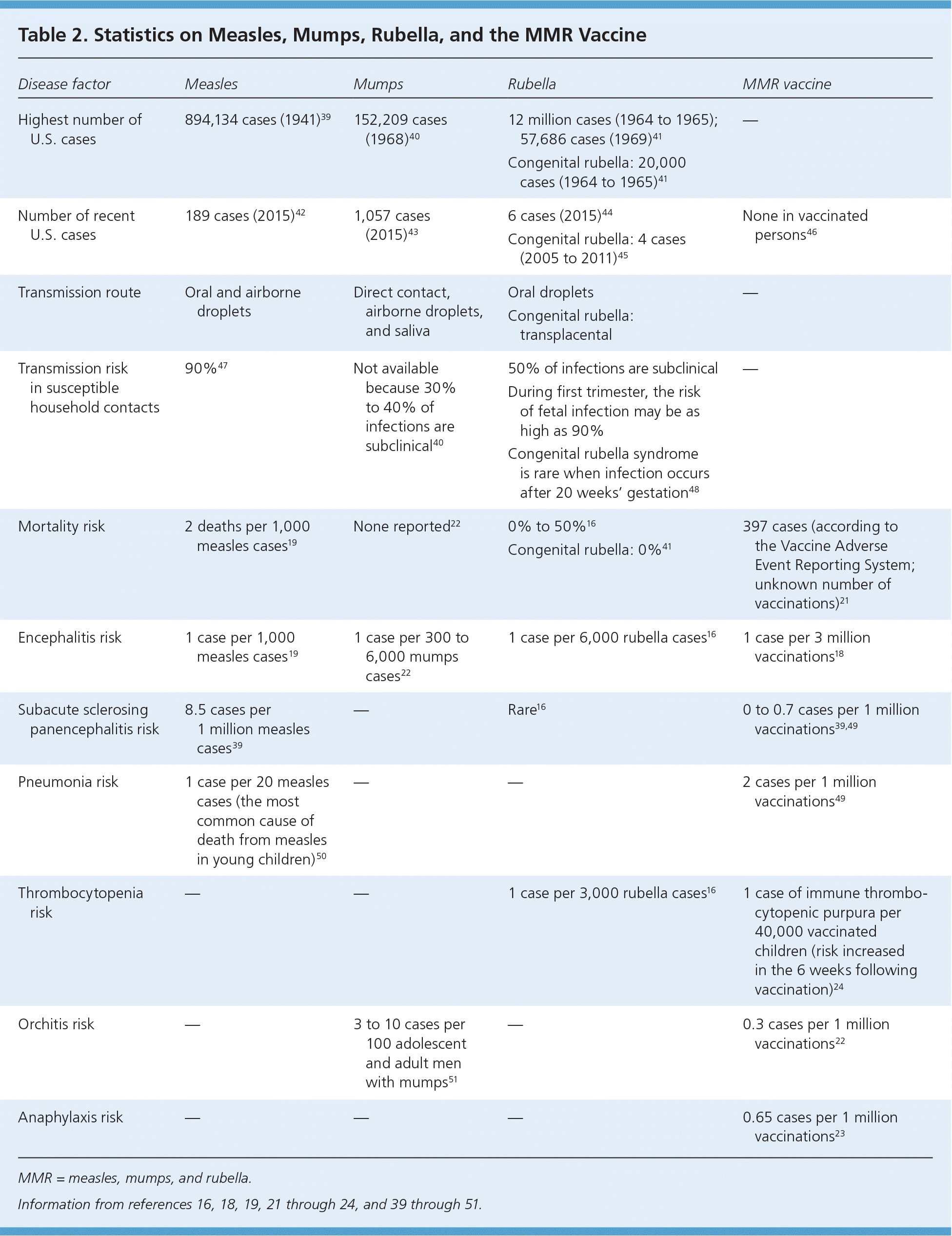
| Disease factor | Measles | Mumps | Rubella | MMR vaccine |
|---|---|---|---|---|
| Highest number of U.S. cases | 894,134 cases (1941)39 | 152,209 cases (1968)40 | 12 million cases (1964 to 1965); 57,686 cases (1969)41 | — |
| Congenital rubella: 20,000 cases (1964 to 1965)41 | ||||
| Number of recent U.S. cases | 189 cases (2015)42 | 1,057 cases (2015)43 | 6 cases (2015)44 | None in vaccinated persons 46 |
| Congenital rubella: 4 cases (2005 to 2011)45 | ||||
| Transmission route | Oral and airborne droplets | Direct contact, airborne droplets, and saliva | Oral droplets | — |
| Congenital rubella: transplacental | ||||
| Transmission risk in susceptible household contacts | 90%47 | Not available because 30% to 40% of infections are subclinical40 | 50% of infections are subclinical | — |
| During first trimester, the risk of fetal infection may be as high as 90% | ||||
| Congenital rubella syndrome is rare when infection occurs after 20 weeks' gestation48 | ||||
| Mortality risk | 2 deaths per 1,000 measles cases19 | None reported22 | 0% to 50%16 | 397 cases (according to the Vaccine Adverse Event Reporting System; unknown number of vaccinations)21 |
| Congenital rubella: 35%41 | ||||
| Encephalitis risk | 1 case per 1,000 measles cases 19 | 1 case per 300 to 6,000 mumps cases 22 | 1 case per 6,000 rubella cases16 | 1 case per 3 million vaccinations18 |
| Subacute sclerosing panencephalitis risk | 8.5 cases per 1 million measles cases 39 | — | Rare16 | 0 to 0.7 cases per 1 million vaccinations39,49 |
| Pneumonia risk | 1 case per 20 measles cases (the most common cause of death from measles in young children)50 | — | — | 2 cases per 1 million vaccinations 49 |
| Thrombocytopenia risk | — | — | 1 case per 3,000 rubella cases16 | 1 case of immune thrombocytopenic purpura per 40,000 vaccinated children (risk increased in the 6 weeks following vaccination)24 |
| Orchitis risk | — | 3 to 10 cases per 100 adolescent and adult men with mumps 51 | — | 0.3 cases per 1 million vaccinations 22 |
| Anaphylaxis risk | — | — | — | 0.65 cases per 1 million vaccinations23 |
HPV Vaccine
HPV is a sexually transmitted infection that can cause genital warts and anogenital and oropharyngeal cancers.52,53 The HPV vaccine has been recommended for all adolescents since 2011.53 However, as of 2013 only 57.3% of girls and 34.6% of boys had received at least one dose.53 HPV immunization rates are suboptimal partly because of unfounded concerns that it encourages sexual activity at a younger age and reduces other protective behaviors.52,54,55 Although hepatitis B can also be sexually transmitted, this vaccine has had near universal uptake. Despite concerns about the safety of the vaccine, the most common adverse effects are transient and similar to those of other vaccines, such as mild pain and bruising at the injection site, headache, lightheadedness, and syncope.53,56,57
Rotavirus Vaccine
The first rotavirus vaccine licensed in the United States was withdrawn because of an increased incidence of intussusception. Children who have received the newer vaccines have only a slightly increased risk of intussusception compared with unvaccinated infants and a lower risk compared to those with rotavirus infection. Intussusception occurs during the time of maximum viral replication after vaccination: three to five days after immunization with the pentavalent vaccine (Rotateq) and four to six days after immunization with the monovalent vaccine (Rotarix).58 The risk of intussusception is about one event per 100,000 vaccine doses.58 Immunizing a birth cohort of 4 million children would avert 255,000 physician visits, 137,000 visits to the emergency department, and 44,000 hospitalizations, and would prevent 13 deaths.59
Meningococcal Vaccine
Universal immunization with MCV4 has been recommended for adolescents since 2005. The initial dose is recommended at 11 to 12 years of age, with a booster at 16 years of age. This vaccine protects against serogroups A, C, W135, and Y. Previous concerns about an increased risk of Guillain-Barré syndrome associated with the vaccine were unfounded.60 The most common adverse effects are fever, headache, injection site erythema, and dizziness. Immunity wanes over time, even after the booster dose.60 There are now two well-tolerated serogroup B meningococcal vaccines that the FDA approved in 2015. Previous vaccines against serogroup B meningitis caused adverse effects from cross allergenicity with antigens in the human central nervous system.61 The CDC's Advisory Committee on Immunization Practices recommends routine use of these vaccines only in high-risk persons and during outbreaks because of the low prevalence of the disease.62 These two meningococcal serogroup B vaccines are not interchangeable.62
Influenza Vaccine and Guillain-Barré Syndrome
The 1976 swine influenza vaccine was associated with a four- to eightfold increased risk of Guillain-Barré syndrome, or an attributable risk of one case per 100,000 persons vaccinated.57,63 A study of the 2009 influenza vaccine did not show a similar association.64 Although the literature is conflicting, the influenza vaccine may at worst slightly increase the risk of Guillain-Barré syndrome: one hospital admission per 1 million vaccinations, or one additional case per 1.25 million vaccinations.57,63,65 Studies suggest that influenza infection has a stronger association with Guillain-Barré syndrome than does the vaccine, so vaccination may actually decrease the overall incidence.57,63
Varicella and Zoster Vaccines
Before routine vaccination, wild-type varicella caused approximately 10,000 hospitalizations and 100 deaths annually.66 All 50 states mandate vaccination of school-aged children with the varicella vaccine.67Adverse effects are generally mild and include erythema and pain at the injection site (Table 314,66,68–76 ). Local reactions occur in 19% of vaccinated children and 24% of vaccinated adolescents and adults (slightly more after the second dose).66 Both the varicella and the zoster vaccines contain the same attenuated Oka varicella strain. A recent Institute of Medicine report supports a causal relationship between varicella vaccination and disseminated Oka strain varicella-zoster infections such as pneumonia, meningitis, or hepatitis, especially in persons with demonstrated immunodeficiencies.27 Adverse events such as anaphylaxis may be related to sensitivity to vaccine components (e.g., gelatin) rather than to the attenuated vaccine virus itself. Nearly one in three unvaccinated persons in the United States will develop herpes zoster, and this risk increases with age.77 In general, zoster vaccine is well tolerated. The most common adverse effects are erythema (36%), pain or tenderness (35%), swelling (26%), and itchiness (7%) at the injection site.76 The benefits of vaccination and rates of adverse events are greater in persons 60 to 69 years of age compared with older adults.77
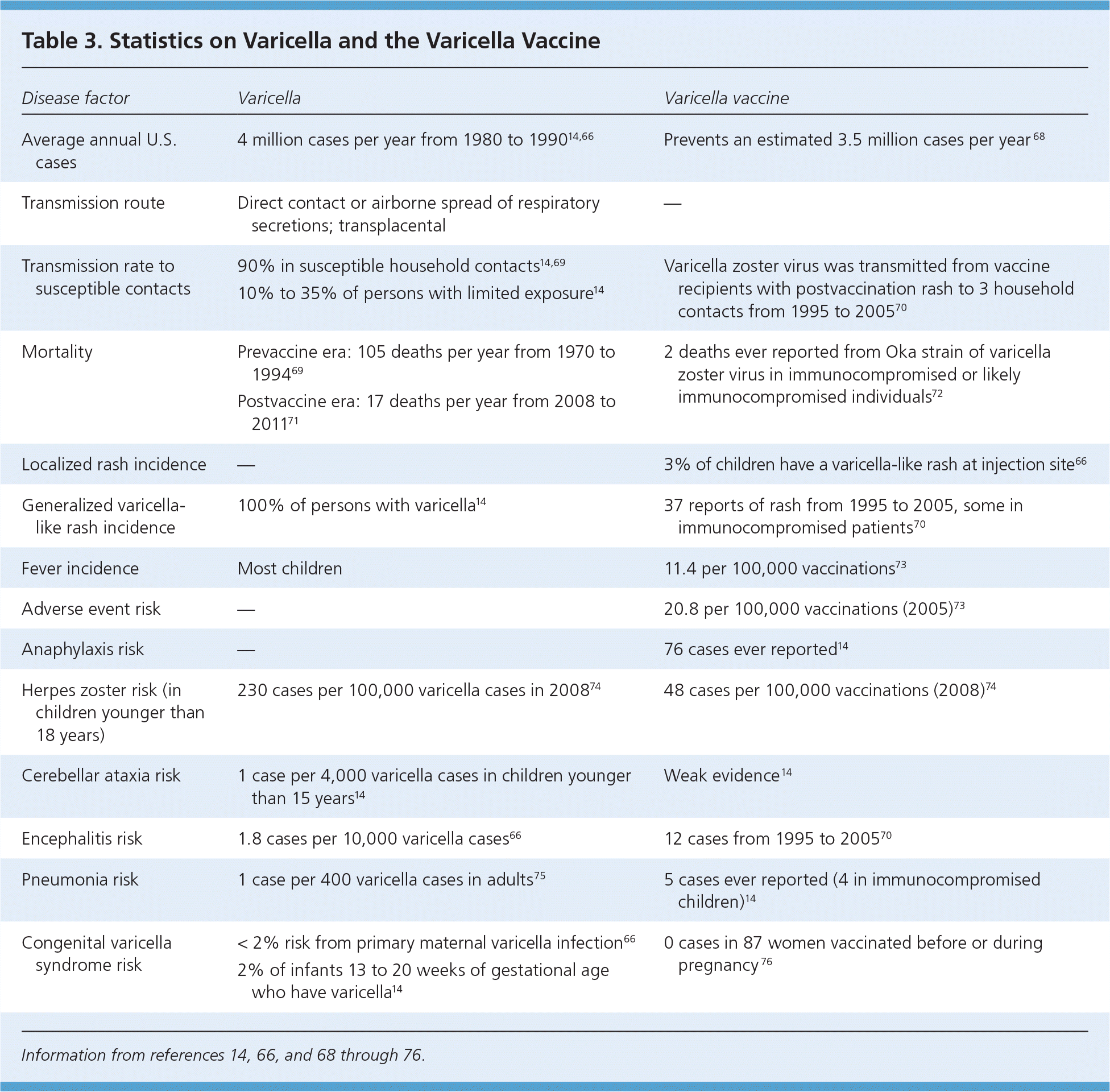
| Disease factor | Varicella | Varicella vaccine |
|---|---|---|
| Average annual U.S. cases | 4 million cases per year from 1980 to 199014,66 | Prevents an estimated 3.5 million cases per year 68 |
| Transmission route | Direct contact or airborne spread of respiratory secretions; transplacental | — |
| Transmission rate to susceptible contacts | 90% in susceptible household contacts14,69 10% to 35% of persons with limited exposure14 | Varicella zoster virus was transmitted from vaccine recipients with postvaccination rash to 3 household contacts from 1995 to 200570 |
| Mortality | Prevaccine era: 105 deaths per year from 1970 to 199469 | 2 deaths ever reported from Oka strain of varicella zoster virus in immunocompromised or likely immunocompromised individuals72 |
| Postvaccine era: 17 deaths per year from 2008 to 201171 | ||
| Localized rash incidence | — | 3% of children have a varicella-like rash at injection site 66 |
| Generalized varicella-like rash incidence | 100% of persons with varicella14 | 37 reports of rash from 1995 to 2005, some in immunocompromised patients70 |
| Fever incidence | Most children | 11.4 per 100,000 vaccinations73 |
| Adverse event risk | — | 20.8 per 100,000 vaccinations (2005)73 |
| Anaphylaxis risk | — | 76 cases ever reported14 |
| Herpes zoster risk (in children younger than 18 years) | 230 cases per 100,000 varicella cases in 200874 | 48 cases per 100,000 vaccinations (2008)74 |
| Cerebellar ataxia risk | 1 case per 4,000 varicella cases in children younger than 15 years14 | Weak evidence 14 |
| Encephalitis risk | 1.8 cases per 10,000 varicella cases66 | 12 cases from 1995 to 200570 |
| Pneumonia risk | 1 case per 400 varicella cases in adults 75 | 5 cases ever reported (4 in immunocompromised children)14 |
| Congenital varicella syndrome risk | < 2% risk from primary maternal varicella infection66 2% of infants 13 to 20 weeks of gestational age who have varicella14 | 0 cases in 87 women vaccinated before or during pregnancy 76 |
Vaccine Refusal
The rate of parental refusal of all or selective immunizations is increasing.78 The American Academy of Family Physicians (AAFP) does not support immunization exemption policies except in cases of allergic or medical contraindication.79 Physicians should provide vaccine information statements to parents who are considering vaccine refusal or delay and direct them to credible sources of information (Table 4).80,81 If parents decide against a recommended vaccine, they should sign a refusal to vaccinate form, and this declination should be documented with provision of the vaccine information statement.82 The American Academy of Pediatrics (AAP) has developed a form that can be used to document vaccine refusal (http://www.aap.org/en-us/Documents/immunization_refusaltovaccinate.pdf). The CDC recommends against dismissing the patient or family from the practice if they refuse vaccination.83 However, the AAP now accepts this practice if done in a conscientious way.84,85
| American Academy of Pediatrics |
| https://www.healthychildren.org/english/safety-prevention/immunizations/pages/default.aspx |
| Centers for Disease Control and Prevention |
| http://www.cdc.gov/vaccines/vac-gen/default.htm |
| Children's Hospital of Philadelphia |
| http://www.chop.edu/centers-programs/parents-pack#.VsIAQLQrJmo |
| Every Child by Two Initiative, Vaccinate Your Family Program |
| http://www.vaccinateyourfamily.org |
| Immunization Action Coalition |
| http://www.vaccineinformation.org |
The AAFP has collaborated with the CDC and the AAP to develop a handout for parents who choose not to vaccinate (http://www.cdc.gov/vaccines/hcp/conversations/downloads/not-vacc-risks-color-office.pdf). Physicians should continue to discuss the benefits of immunizations at subsequent visits because some parents may reconsider the decision not to vaccinate, and some simply postpone certain vaccinations.
Consequences of Failure to Vaccinate
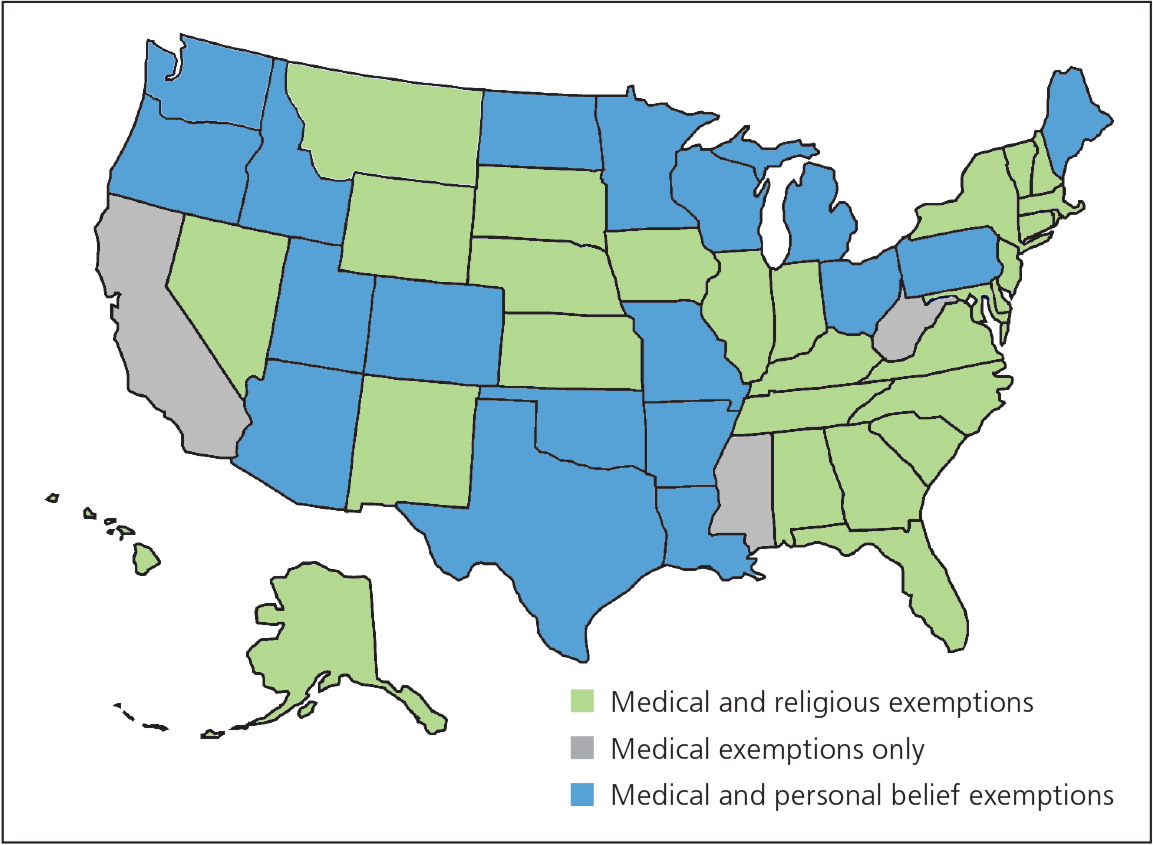
In 2014, there were 667 cases of measles in the United States, mainly within an unvaccinated Amish community.20 In 2012, the largest U.S. pertussis outbreak since 1955 occurred, including 48,277 cases and 20 pertussis-related deaths.86 Pertussis cases have been linked to multiple causes, including waning immunity to the vaccine and children who are unvaccinated under non-medical exemption.87–89 The choice of some parents not to immunize increases the infection risk for all children, including those who are immunized. Sustained community-level transmission can occur in the presence of imperfect vaccine effectiveness and waning immunity, putting those who are susceptible—especially young infants and persons with medical contraindications—at increased risk.87 In the absence of a direct threat from disease, it is clear that some patients and parents will not participate in vaccination programs unless absolute safety can be assured. Full vaccination of students enhances the safety of all, but states vary in regard to acceptable reasons for parental vaccination refusal (Figure 1).90
Vaccine Adverse Event Reporting System
The FDA and the CDC maintain the Vaccine Adverse Event Reporting System (VAERS) as a postmarketing safety surveillance program for adverse events that occur after immunization. Vaccine manufacturers and health care professionals are required to submit potential adverse events.91 VAERS receives approximately 30,000 reports each year. The effectiveness of VAERS as an early warning system was demonstrated by reports of an increased incidence of intussusception after immunization with rotavirus vaccine.91 In 1990, the Vaccine Safety Datalink, a collaboration between the CDC and nine health care organizations, was developed to investigate rare and serious adverse effects reported in the medical literature and to VAERS. The program monitors vaccine safety when a new vaccine is approved or when there are new vaccine recommendations.92
National Vaccine Injury Compensation Program
The National Childhood Vaccine Injury Act of 1986 established the no-fault National Vaccine Injury Compensation Program to compensate patients and families injured by recommended vaccines. This law requires physicians who administer vaccines to document the provision of current vaccine information statements along with the vaccine manufacturer and lot number. The program's website includes a table of covered vaccine injuries.93 Injuries outside those listed and suspected to be vaccine related can also be compensated but require additional evidence. The program is funded by an excise tax on vaccines.
Data Sources: Essential Evidence Plus was searched using the key word vaccine. PubMed was also searched using the key words vaccine adverse effects and thimerosal. Searches were also performed for individual vaccines, and the websites of the Centers for Disease Control and Prevention, the American Academy of Pediatrics, the American Academy of Family Physicians, the Vaccine Adverse Event Reporting System, and the Health Resources and Services Administration were also searched. Search dates: October 28, 2015, to January 17, 2017.
The authors thank Kaitlyn Thomas for assistance with the preparation of the manuscript.