Am Fam Physician. 2017;96(4):257-258
Author disclosure: No relevant financial affiliations.
Lixisenatide (Adlyxin) is a glucagon-like peptide 1 (GLP-1) receptor agonist labeled for the treatment of type 2 diabetes mellitus in adults.1
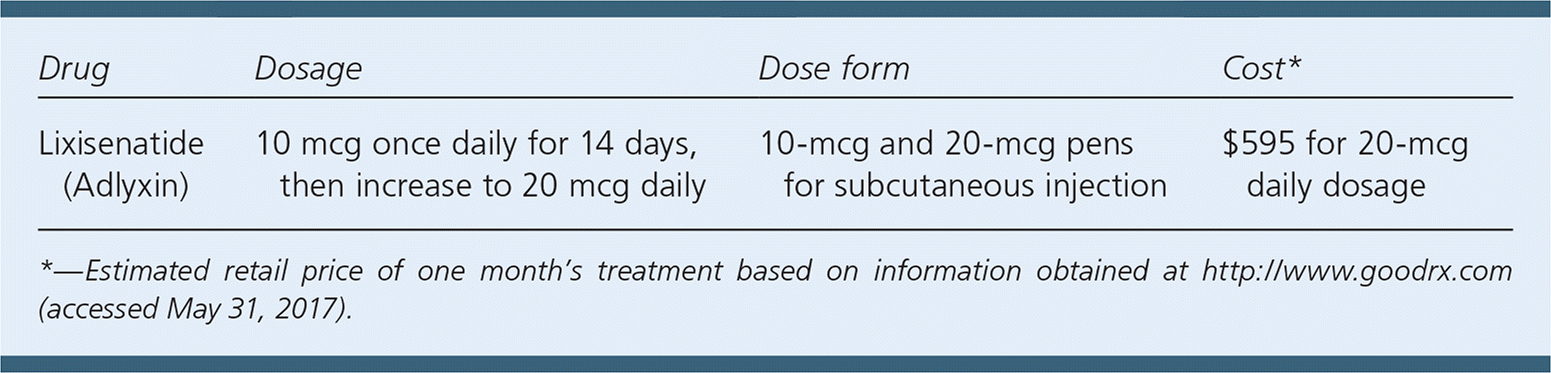
| Drug | Dosage | Dose form | Cost* |
|---|---|---|---|
| Lixisenatide (Adlyxin) | 10 mcg once daily for 14 days, then increase to 20 mcg daily | 10-mcg and 20-mcg pens for subcutaneous injection | $595 for 20-mcg daily dosage |
SAFETY
Lixisenatide is not recommended for patients with type 1 diabetes, a history of chronic or unexplained pancreatitis, diabetic ketoacidosis, gastroparesis, or end-stage renal disease.1 Acute pancreatitis has been reported with the use of other GLP-1 receptor agonists and in initial studies of lixisenatide.1 Adverse effects increase with poor renal function, and lixisenatide labeling recommends against use by patients with an estimated glomerular filtration rate less than 15 mL per minute per 1.73 m2.1 Patients with an estimated glomerular filtration rate less than 60 mL per minute per 1.73 m2 should be monitored closely for gastrointestinal and hypoglycemic adverse events.1
Adverse effects of lixisenatide are similar to those of other GLP-1 receptor agonists.1 Severe symptomatic hypoglycemia, defined as an event requiring assistance or medical intervention, or with a serum blood glucose level less than 36 mg per dL (2.0 mmol per L), occurred in less than 1% of patients using lixisenatide over a 12- to 24-week period.1 The risk of symptomatic hypoglycemia, defined as clinical symptoms resulting from a plasma glucose level less than 60 mg per dL (3.3 mmol per L), increases significantly when lixisenatide is combined with basal insulin with or without a sulfonylurea (47% vs. 22%; number needed to harm [NNH] = 4). Symptomatic hypoglycemia occurs less frequently when lixisenatide is combined with metformin alone (1% vs. 3%; NNH = 50). Lixisenatide does not appear to increase the risk of cardiovascular morbidity or mortality over two years when used in adults with type 2 diabetes following a recent acute coronary event.2 Lixisenatide has not been studied in pregnant women, and it is unknown whether it is excreted in breast milk.1
TOLERABILITY
Gastrointestinal effects, including nausea (25%) and vomiting (10%), occur in more than one-half of patients at the start of therapy, likely due to a delayed emptying effect.3 Patients may have difficulty tolerating lixisenatide in the first few weeks of therapy, but they may become accustomed to the adverse effects over time.3 Headache and dizziness have also been reported.1 In clinical studies, 3.3% of patients discontinued treatment because of adverse effects.3 Patients should be counseled to consume less food initially and at a slower-than-normal pace to minimize the risk of gastrointestinal adverse effects.
EFFECTIVENESS
Lixisenatide reduces A1C by 0.46% when added to metformin, 0.58% when added to a sulfonylurea, and 0.36% when combined with basal insulin with or without metformin.1 When added to metformin, lixisenatide will increase the percentage of patients achieving an A1C less than 7% compared with metformin alone (44% vs. 22%; number needed to treat = 5).1 When combined with metformin, lixisenatide produces a reduction in A1C similar to that of exenatide (Byetta). Patients may experience weight loss of approximately 4 lb (2 kg), although it is unclear whether this effect persists after 24 weeks.1 Importantly, lixisenatide has not been evaluated to determine its long-term effect on diabetes-related morbidity or on all-cause mortality.
PRICE
The cost of a one-month supply of lixisenatide is approximately $595. This is similar to the price of other GLP-1 receptor agonists for the treatment of type 2 diabetes.
SIMPLICITY
Lixisenatide is a once-daily subcutaneous injection (initial dose of 10 mcg) that requires a dose increase (to 20 mcg daily) after 14 days. Each prefilled pen provides 14 set doses.1 Patients should administer the injection within one hour of the first meal of the day.1 The medication administration process is similar to that of other GLP-1 receptor agonists, although several GLP-1 receptor agonists allow for once-weekly dosing. There is no dosage adjustment necessary for older adults, patients with hepatic dysfunction, or patients with mild to moderate renal impairment, although close monitoring in these patients is recommended.1
Bottom Line
Lixisenatide is an easy-to-use, once-daily injectable therapy for the treatment of type 2 diabetes in adults. It is at least as effective as exenatide at reducing A1C and body weight, with similar rates of gastrointestinal adverse effects. Lixisenatide could be considered a second-line therapy in addition to metformin; however, it may be more appropriate as a third-line option, given its high rates of hypoglycemia and gastrointestinal intolerability. Liraglutide (Victoza) may be a more preferable GLP-1 receptor agonist because of its proven ability to reduce cardiovascular events.4