
Am Fam Physician. 2017;96(5):online

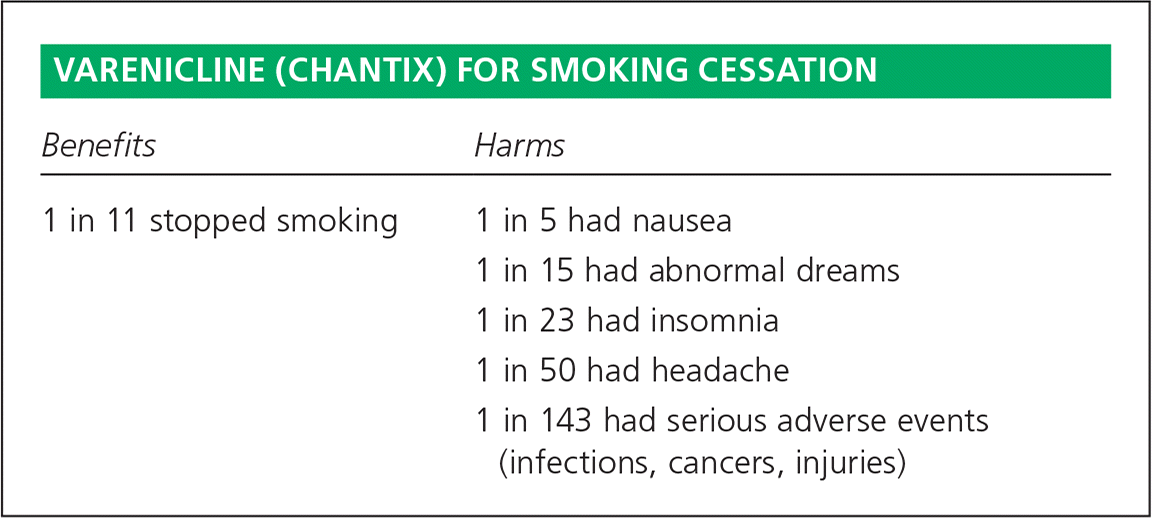
| Benefits | Harms |
|---|---|
| 1 in 11 stopped smoking | 1 in 5 had nausea |
| 1 in 15 had abnormal dreams | |
| 1 in 23 had insomnia | |
| 1 in 50 had headache | |
| 1 in 143 had serious adverse events (infections, cancers, injuries) |
Details for This Review
Study Population: Adult smokers willing to attempt quitting smoking
Efficacy End Points: Smoking cessation at least six months from the start of intervention
Harm End Points: Medication side effects or adverse events
Narrative: Worldwide, smoking remains the main preventable cause of morbidity and premature death. Dependence on nicotine reflects the effects of the drug on neuronal receptors in the brain, which stimulate the release of dopamine. Varenicline (Chantix) is a medication that aids in smoking cessation by acting as a nicotine receptor partial agonist, and is one of seven first-line medications recommended to increase long-term smoking abstinence.1 The authors of a systematic Cochrane review performed a meta-analysis examining 39 randomized trials (N = 11,801) that tested varenicline in a variety of populations and settings, and against various comparators.2 Trials included in the review variably enrolled adult smokers with the goal of cessation for at least six months.
Varenicline adminstered at the standard dosage (1 mg twice daily) more than doubled the chances of quitting compared with placebo, with a pooled risk ratio [RR] of 2.24 (95% confidence interval [CI], 2.06 to 2.43; 27 trials, N = 12,625; high-quality evidence). Varenicline at a reduced dosage (0.5 mg twice daily) remained effective with an RR of 2.08 (95% CI, 1.56 to 2.78; four trials, N = 1,266), a cessation rate that is similar to varenicline vs. bupropion (Zyban), with an RR of 1.39 (95% CI, 1.25 to 1.54; five trials, N = 5,877; high-quality evidence), and nicotine replacement therapy, with an RR of 1.25 (95% CI, 1.14 to 1.37; eight trials, N = 6,624; moderate-quality evidence). Based on this evidence and using advanced statistical modeling, the authors calculated the number needed to treat (NNT) with varenicline as 11 (95% CI, 9 to 13). Using the compiled data and absolute risk reduction without advanced statistics gives an NNT for varenicline vs. placebo, bupropion, and nicotine replacement therapy of 6, 15, and 20, respectively.
The predominant adverse event was mild to moderate nausea subsiding over time, with rates between 6% and 51%. Rates of serious adverse events were noted to be about 25% higher in those receiving varenicline compared with the control groups, with an RR of 1.25 (95% CI, 1.04 to 1.49; 29 studies, N = 15,370; I2 = 0%), and included comorbidities such as infections, cancers, and injuries, most of which were considered to be unrelated to the treatment. The number needed to harm (NNH) for one serious adverse event as calculated by the authors using advanced statistical modeling is 143 (95% CI, 74 to 556). Without advanced statistical methods and using the compiled data resulted in an NNH of 165.
Caveats: In 2009, the U.S. Food and Drug Administration (FDA) added a black-box warning to varenicline because of post-marketing safety reports of an association between varenicline and psychiatric adverse events including depressed mood, agitation, and suicidal behavior/ideation.3 The FDA required Pfizer, the manufacturer of Chantix, to conduct a clinical trial to evaluate the risk of neuropsychiatric adverse events, and found the risk to be lower than previously suspected. As a result, the FDA removed the black-box warning for serious mental health side effects from the Chantix drug label on December 16, 2016.
In 2011, the FDA advised that varenicline might slightly increase cardiovascular events in persons with cardiovascular disease.3 Results are pending from a large trial that should give more definitive data on this issue. Should data become available suggesting a significant increase in major adverse cardiac events, it would necessitate revisiting this topic and potentially changing the recommendation. A 2015 update from the FDA added a warning that varenicline may change the way people react to alcohol (e.g., possible increased drunkenness, unusual behavior, memory lapse), as well as rare accounts of seizures with treatment. These warnings were added after a review of the FDA Adverse Event Reporting System and a case series submitted by Pfizer. The actual NNT and NNH vary depending on how the calculation is performed. However, calculations without advanced statistical modeling are close to those reported by the authors, and show varenicline to be beneficial.
The serious adverse events data do not differentiate between events caused by treatment vs. comorbidities. Also, losses to follow-up were higher in the control groups (mean of 28.4%) than in the treatment groups (mean of 23.8%) of most of the studies (25 of 29), which may underestimate serious adverse event rates in the control groups. Thus, serious adverse event rates and associated NNH attributable to varenicline are likely overestimated.
Copyright 2015 The NNT Group (theNNT.com). Used with permission.
This series is coordinated by Dean A. Seehusen, MD, MPH, AFP Contributing Editor, and Daniel Runde, MD, from the NNT Group.
Author disclosure: No relevant financial affiliations.
The opinions and assertions contained herein are the private views of the authors and are not to be construed as official or as reflecting the views of the U.S. Air Force Medical Department or the U.S. Air Force at large.
This review is available from the NNT Group at http://www.thennt.com/nnt/varenicline-smoking-cessation/.
