Am Fam Physician. 2017;96(8):540-542
Author disclosure: No relevant financial affiliations.
Dulaglutide (Trulicity) is one of six marketed glucagon-like peptide-1 (GLP-1) agonists for the treatment of type 2 diabetes mellitus. In addition to diet and exercise, it may be used alone or in combination with other diabetes medications. It lowers blood glucose levels by stimulating synthesis and secretion of insulin and inhibiting secretion of glucagon.1
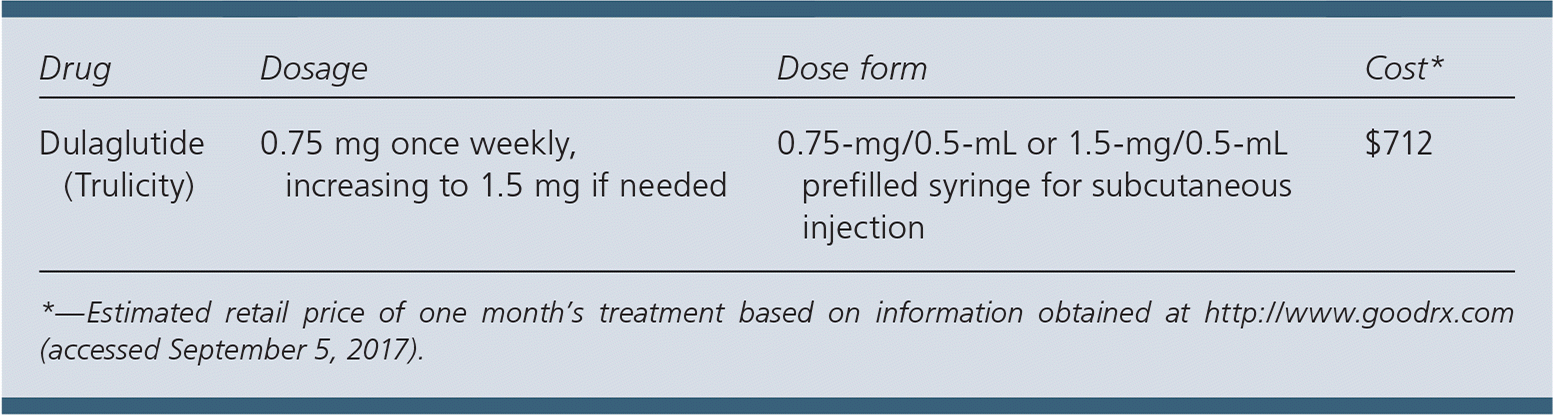
| Drug | Dosage | Dose form | Cost* |
|---|---|---|---|
| Dulaglutide (Trulicity) | 0.75 mg once weekly, increasing to 1.5 mg if needed | 0.75-mg/0.5-mL or 1.5-mg/0.5-mL prefilled syringe for subcutaneous injection | $712 |
SAFETY
Dulaglutide does not appear to increase cardiovascular events or mortality compared with placebo or other therapies for diabetes, including metformin, sitagliptin (Januvia), exenatide (Byetta), and glargine insulin (Lantus). Safety studies have evaluated the use of dulaglutide in patients for one to two years.2 In one study, 376 patients out of 657 receiving dulaglutide completed a two-year follow-up.3 No difference in mortality or cardiovascular events was reported between patients who received dulaglutide and those who did not.
The risk of hypoglycemia (defined as a serum blood glucose level less than 70 mg per dL [3.9 mmol per L]) with dulaglutide alone1,4 or in combination with metformin is about 8% over one year.3 Episodes of severe hypoglycemia (requiring intervention) are less common, occurring in about 0.2% of patients over one to two years.4 In clinical trials, all episodes of severe hypoglycemia with dulaglutide use occurred in the setting of concomitant sulfonylurea or insulin therapy.4–6 The risk of hypoglycemia with dulaglutide does not appear to be dose dependent.1
One of the biggest concerns about dulaglutide and other GLP-1 agonists from postmarketing reports is the risk of pancreatitis. These products contain labeling recommending against their use in patients with a history of pancreatitis.7 An independent meta-analysis of 55 randomized controlled trials following more than 33,000 patients for six months to four years found the risk of pancreatitis to be 0.1% in patients using any class of medication for type 2 diabetes.8 There was no difference in risk between GLP-1 agonists and other classes.
As with other GLP-1 agonists, there is a theoretical risk of patients developing thyroid C-cell carcinoma, based on rodent studies. These products should not be used in patients with a personal or family history of medullary thyroid carcinoma or multiple endocrine neoplasia syndrome type 2.7
Dulaglutide is not recommended for use in children. Animal studies suggest a potential risk to the fetus if the medication is used during pregnancy. It has not been evaluated in breastfeeding women.7
TOLERABILITY
Dulaglutide is generally well tolerated. In clinical trials, dropout rates were similar to those with placebo. About 10% of patients will experience nausea, vomiting, abdominal pain, decreased appetite, and diarrhea. These adverse effects are most common during the first two weeks of therapy. Vomiting is the only adverse effect that is significantly increased compared with placebo or metformin monotherapy (number needed to harm = 21).1
EFFECTIVENESS
No studies have evaluated the effect of dulaglutide on cardiovascular events, morbidity, or mortality in patients with type 2 diabetes.
PRICE
A one-month supply of dulaglutide costs approximately $712, which is similar to the price of other GLP-1 agonists. In contrast, other glucose-lowering agents used as monotherapy are much less expensive.
SIMPLICITY
Dulaglutide is a once-weekly subcutaneous injection. It comes in a single-dose 0.75-mg/0.5-mL prefilled syringe that should be stored in a refrigerator until use. The recommended starting dosage is 0.75 mg once weekly, increasing to 1.5 mg once weekly if additional glycemic control is needed.
Bottom Line
Dulaglutide is an easy-to-use, once-weekly injectable therapy for the treatment of type 2 diabetes in adults. It produces a modest decrease in A1C levels with only a small risk of severe hypoglycemia. The small average weight loss it induces may be an advantage for some patients. Critically, there is no research showing a mortality or morbidity benefit with dulaglutide. It may be used as adjuvant therapy to metformin, but there are less expensive, equally effective options available when adding a second medication for patients with type 2 diabetes. Liraglutide (Victoza) may be a preferable GLP-1 agonist, given its ability to reduce cardiovascular events.10