Am Fam Physician. 2017;96(8):545-546
Author disclosure: No relevant financial affiliations.
Key Points for Practice
• All persons older than six months without a contraindication should receive annual influenza vaccination. There is no recommendation for a specific vaccine in persons for whom more than one licensed product is available.
• Pregnant women may receive any licensed, age-appropriate vaccine that is not live.
• Again this season, live attenuated influenza vaccine is not recommended because of its previous low effectiveness against influenza A(H1N1)pdm09.
From the AFP Editors
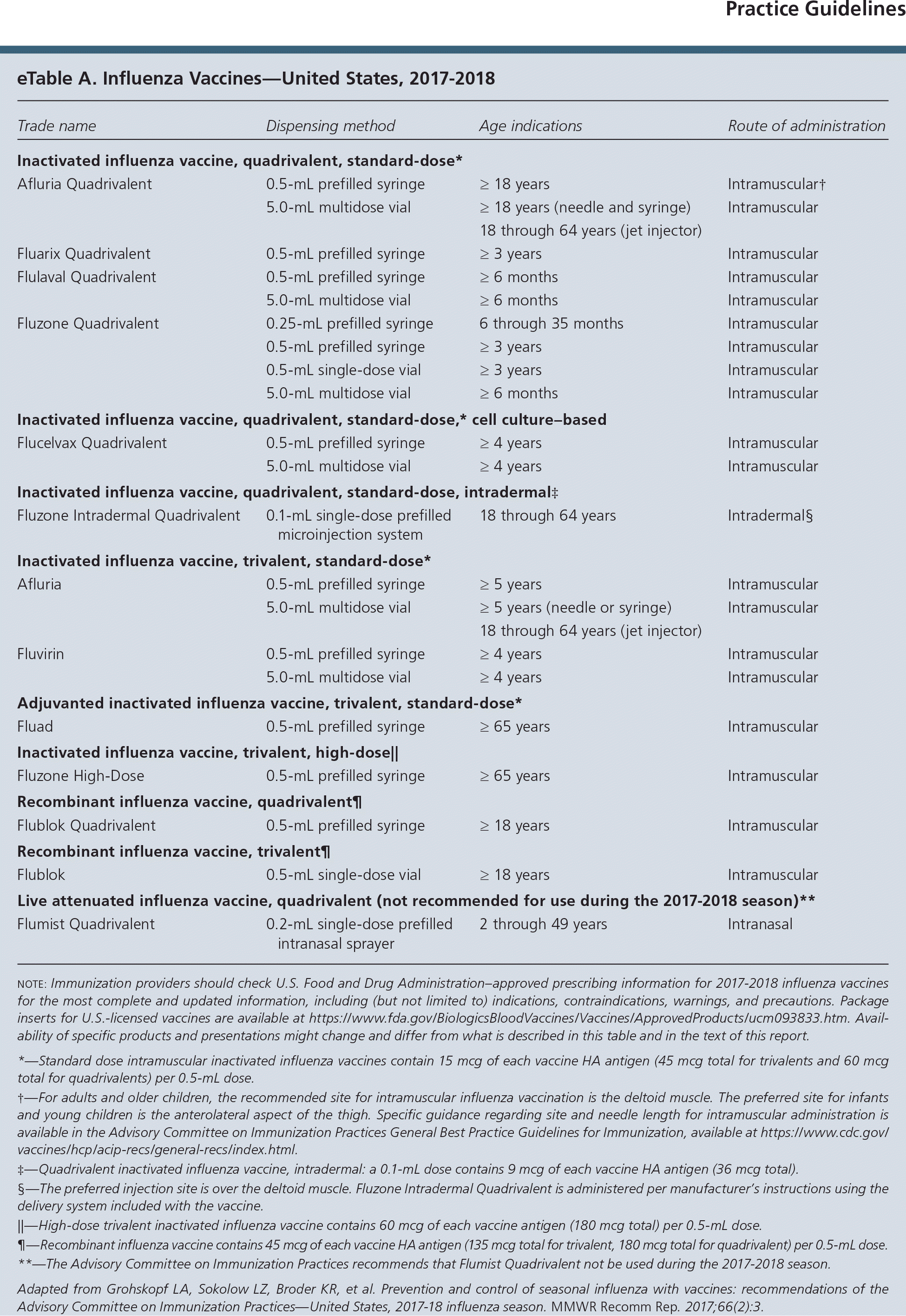
The Centers for Disease Control and Prevention's Advisory Committee on Immunization Practices (ACIP) has released its recommendations on influenza vaccination for the 2017–2018 season. In this update, ACIP announces the currently available vaccine products (eTable A), reviews license and labeling changes, and issues recommendations for specific populations. A summary of ACIP's seasonal influenza vaccine recommendations is available at https://www.cdc.gov/flu/professionals/acip/index.htm.
| Trade name | Dispensing method | Age indications | Route of administration |
|---|---|---|---|
| Inactivated influenza vaccine, quadrivalent, standard-dose* | |||
| Afluria Quadrivalent | 0.5-mL prefilled syringe | ≥ 18 years | Intramuscular† |
| 5.0-mL multidose vial | ≥ 18 years (needle and syringe) | Intramuscular | |
| 18 through 64 years (jet injector) | |||
| Fluarix Quadrivalent | 0.5-mL prefilled syringe | ≥ 3 years | Intramuscular |
| Flulaval Quadrivalent | 0.5-mL prefilled syringe | ≥ 6 months | Intramuscular |
| 5.0-mL multidose vial | ≥ 6 months | Intramuscular | |
| Fluzone Quadrivalent | 0.25-mL prefilled syringe | 6 through 35 months | Intramuscular |
| 0.5-mL prefilled syringe | ≥ 3 years | Intramuscular | |
| 0.5-mL single-dose vial | ≥ 3 years | Intramuscular | |
| 5.0-mL multidose vial | ≥ 6 months | Intramuscular | |
| Inactivated influenza vaccine, quadrivalent, standard-dose,* cell culture–based | |||
| Flucelvax Quadrivalent | 0.5-mL prefilled syringe | ≥ 4 years | Intramuscular |
| 5.0-mL multidose vial | ≥ 4 years | Intramuscular | |
| Inactivated influenza vaccine, quadrivalent, standard-dose, intradermal‡ | |||
| Fluzone Intradermal Quadrivalent | 0.1-mL single-dose prefilled microinjection system | 18 through 64 years | Intradermal§ |
| Inactivated influenza vaccine, trivalent, standard-dose* | |||
| Afluria | 0.5-mL prefilled syringe | ≥ 5 years | Intramuscular |
| 5.0-mL multidose vial | ≥ 5 years (needle or syringe) | Intramuscular | |
| 18 through 64 years (jet injector) | |||
| Fluvirin | 0.5-mL prefilled syringe | ≥ 4 years | Intramuscular |
| 5.0-mL multidose vial | ≥ 4 years | Intramuscular | |
| Adjuvanted inactivated influenza vaccine, trivalent, standard-dose* | |||
| Fluad | 0.5-mL prefilled syringe | ≥ 65 years | Intramuscular |
| Inactivated influenza vaccine, trivalent, high-dose|| | |||
| Fluzone High-Dose | 0.5-mL prefilled syringe | ≥ 65 years | Intramuscular |
| Recombinant influenza vaccine, quadrivalent¶ | |||
| Flublok Quadrivalent | 0.5-mL prefilled syringe | ≥ 18 years | Intramuscular |
| Recombinant influenza vaccine, trivalent¶ | |||
| Flublok | 0.5-mL single-dose vial | ≥ 18 years | Intramuscular |
| Live attenuated influenza vaccine, quadrivalent (not recommended for use during the 2017–2018 season)** | |||
| Flumist Quadrivalent | 0.2-mL single-dose prefilled intranasal sprayer | 2 through 49 years | Intranasal |
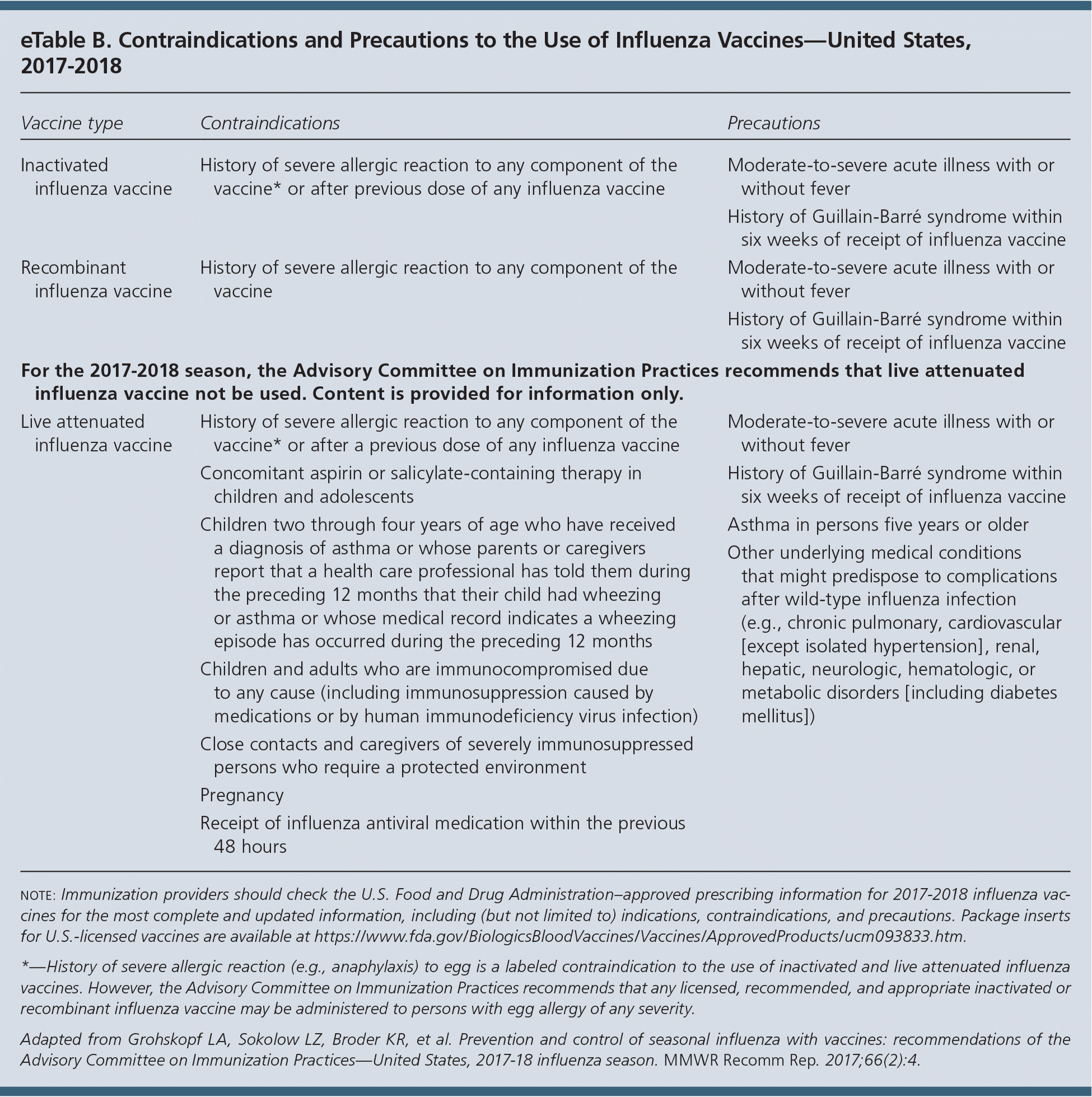
ACIP recommends that all persons older than six months without a contraindication receive annual influenza vaccination. There is no recommendation for a specific vaccine in persons for whom more than one licensed product is available. The updated recommendations state that pregnant women may receive any licensed, age-appropriate vaccine that is not live. Contraindications and precautions to the influenza vaccines are listed in eTable B.
| Vaccine type | Contraindications | Precautions |
|---|---|---|
| Inactivated influenza vaccine |
|
|
| Recombinant influenza vaccine |
|
|
| For the 2017–2018 season, the Advisory Committee on Immunization Practices recommends that live attenuated influenza vaccine not be used. Content is provided for information only. | ||
| Live attenuated influenza vaccine |
|
|
This season's available vaccine products include inactivated influenza vaccines in trivalent and quadrivalent formulations, and recombinant influenza vaccine in trivalent and quadrivalent formulations. The three viruses in this season's trivalent influenza vaccines include an A/Michigan/45/2015 (H1N1)pdm09–like virus, an A/Hong Kong/4801/2014 (H3N2)–like virus, and a B/Brisbane/60/2008–like virus (Victoria lineage). The quadrivalent vaccines include these three viruses plus a B/Phuket/3072/2013–like virus (Yamagata lineage). Afluria, a trivalent inactivated influenza vaccine, is now approved in persons five years or older, consistent with the U.S. Food and Drug Administration's labeling.
As in the 2016–2017 season, live attenuated influenza vaccine (LAIV4; Flumist Quadrivalent) is not recommended because of its low effectiveness against influenza A(H1N1) pdm09 in the United States. The 2017–2018 ACIP report mentions LAIV for informational purposes only.
Recommendations for Specific Populations
PERSONS AT HIGH RISK OF MEDICAL COMPLICATIONS AND THEIR CAREGIVERS
Vaccination is especially important in persons at increased risk of medical complications from influenza and of influenza-related outpatient, emergency department, or hospital visits. In cases of a limited vaccine supply, priority should be given to the following groups:
Children six through 59 months of age
Adults 50 years and older
Adults and children with chronic pulmonary (e.g., asthma) or cardiovascular (not including isolated hypertension), renal, hepatic, neurologic, hematologic, or metabolic disorders (e.g., diabetes mellitus)
Persons who are immunocompromised (e.g., from medications or human immunodeficiency virus infection)
Women who are pregnant or will be pregnant during the influenza season
Children and adolescents (six months through 18 years of age) who are receiving aspirin- or salicylate-containing medications and who may be at risk of Reye syndrome after influenza virus infection
Residents of nursing homes or long-term care facilities
American Indians and Alaska Natives
Persons with a body mass index of 40 kg per m2 or greater.
Although LAIV4 is not recommended during the 2017–2018 season, health care professionals who choose to use it should follow guidance for the use of LAIV4 for high-risk persons (eTable B). LAIV4 should not be used in persons with most forms of altered immunocompetence because of the possible risk of disease attributable to the vaccine virus. Additionally, it should not be used in pregnant women because it is a live virus.
Persons who live with or care for persons at higher risk of influenza-related complications should also be prioritized for vaccination. These include health care personnel in inpatient and outpatient care settings; employees of nursing homes or long-term care facilities who have contact with patients or residents; students who have contact with patients; household contacts (including children) and caregivers of children younger than five years or adults 50 years or older; and household contacts and caregivers of persons with medical conditions that put them at high risk of complications from influenza.
PERSONS WITH A HISTORY OF GUILLAIN-BARRÉ SYNDROME
A history of Guillain-Barré syndrome within six weeks after receiving any influenza vaccine is a precaution to vaccination. If not at high-risk of complications, these individuals generally should not be vaccinated. Influenza antiviral chemoprophylaxis may be considered. If persons with a history of Guillain-Barré are at high risk of complications from influenza, the benefits of vaccination may outweigh the risks.
PERSONS WITH A HISTORY OF EGG ALLERGY
Persons who have experienced only hives after exposure to egg should receive the influenza vaccine. Any licensed and recommended influenza vaccine (i.e., any inactivated influenza vaccine or recombinant influenza vaccine) that is otherwise appropriate for the individual may be used. Persons who have experienced more severe reactions (e.g., angioedema, respiratory distress, lightheadedness, recurrent emesis) or who required epinephrine or emergency medical intervention after exposure to egg may also receive any licensed and recommended influenza vaccine. These individuals should receive vaccination in an inpatient or outpatient setting under supervision of a clinician able to recognize and manage severe allergic reaction.
Persons who have previously experienced a severe allergic reaction to the influenza vaccine, regardless of the suspected component, should not receive the vaccine. Although a period of observation following vaccination is not recommended for persons with egg allergy, ACIP recommends that clinicians observe patients for 15 minutes after administration of any vaccine to decrease the risk of injury in case of syncope.
Guideline source: Advisory Committee on Immunization Practices
Evidence rating system used? No
Literature search described? No
Guideline developed by participants without relevant financial ties to industry? Not reported
Published source:MMWR Morb Mortal Wkly Rep. August 25, 2017;66(2):1–24
Available at:https://www.cdc.gov/mmwr/volumes/66/rr/rr6602a1.htm
MARA LAMBERT, AFP Senior Associate Editor