Am Fam Physician. 2018;97(2):online
Related Putting Prevention into Practice: Screening for Preeclampsia.
As published by the U.S. Preventive Services Task Force.
Summary of Recommendation and Evidence
The USPSTF recommends screening for preeclampsia in pregnant women with blood pressure measurements throughout pregnancy (Table 1). B recommendation.
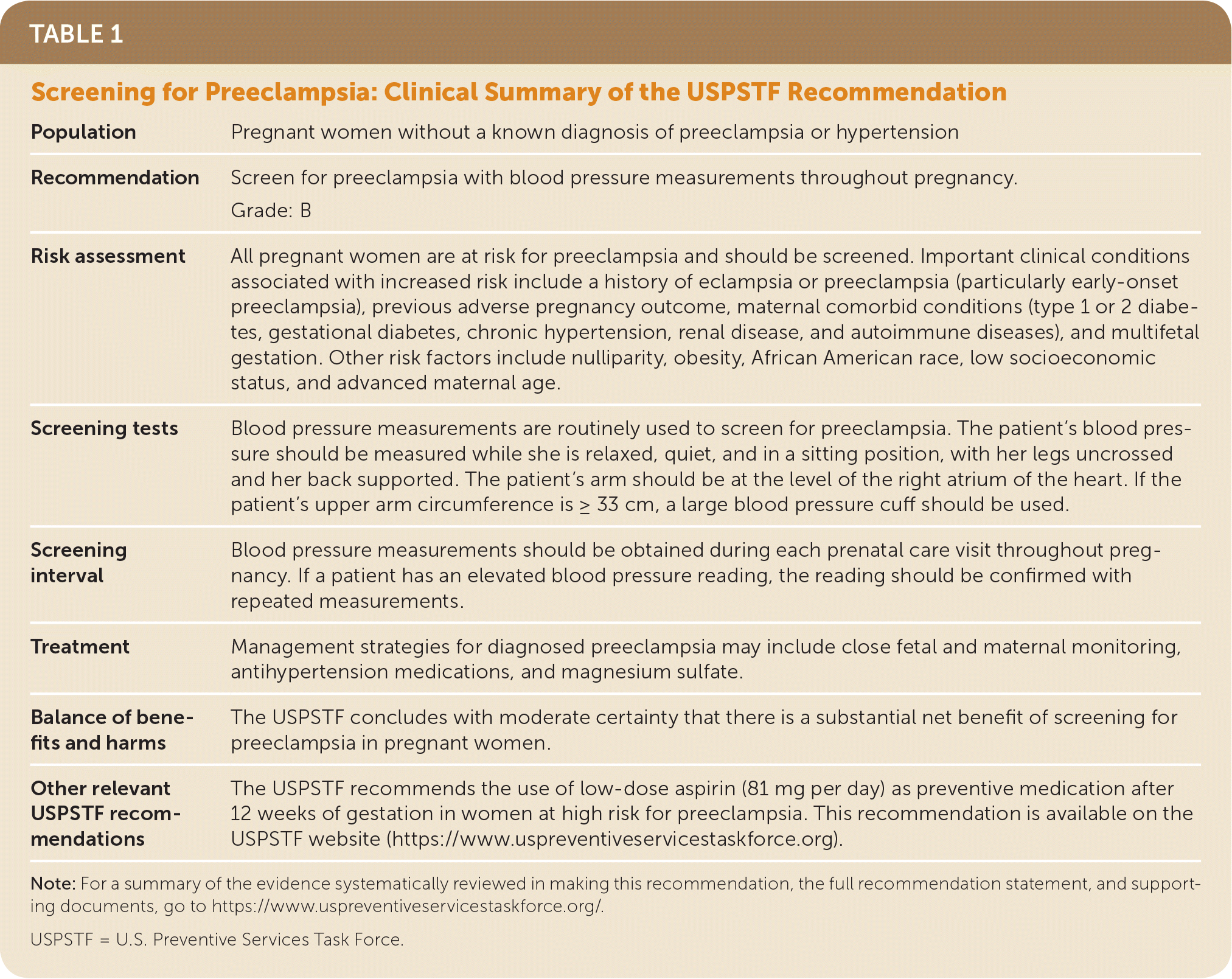
| Population | Pregnant women without a known diagnosis of preeclampsia or hypertension |
| Recommendation | Screen for preeclampsia with blood pressure measurements throughout pregnancy. Grade: B |
| Risk assessment | All pregnant women are at risk for preeclampsia and should be screened. Important clinical conditions associated with increased risk include a history of eclampsia or preeclampsia (particularly early-onset preeclampsia), previous adverse pregnancy outcome, maternal comorbid conditions (type 1 or 2 diabetes, gestational diabetes, chronic hypertension, renal disease, and autoimmune diseases), and multifetal gestation. Other risk factors include nulliparity, obesity, African American race, low socioeconomic status, and advanced maternal age. |
| Screening tests | Blood pressure measurements are routinely used to screen for preeclampsia. The patient's blood pressure should be measured while she is relaxed, quiet, and in a sitting position, with her legs uncrossed and her back supported. The patient's arm should be at the level of the right atrium of the heart. If the patient's upper arm circumference is = 33 cm, a large blood pressure cuff should be used. |
| Screening interval | Blood pressure measurements should be obtained during each prenatal care visit throughout pregnancy. If a patient has an elevated blood pressure reading, the reading should be confirmed with repeated measurements. |
| Treatment | Management strategies for diagnosed preeclampsia may include close fetal and maternal monitoring, antihypertension medications, and magnesium sulfate. |
| Balance of benefits and harms | The USPSTF concludes with moderate certainty that there is a substantial net benefit of screening for preeclampsia in pregnant women. |
| Other relevant USPSTF recommendations | The USPSTF recommends the use of low-dose aspirin (81 mg per day) as preventive medication after 12 weeks of gestation in women at high risk for preeclampsia. This recommendation is available on the USPSTF website (https://www.uspreventiveservicestaskforce.org). |
Rationale
IMPORTANCE
Preeclampsia, a relatively common hypertensive disorder occurring during pregnancy, affects approximately 4% of pregnancies in the United States.1 It has multiple subtypes and potentially serious, even fatal health outcomes.2,3 Although pregnant women can have other hypertensive conditions along with preeclampsia, preeclampsia is defined as new-onset hypertension (or, in patients with existing hypertension, worsening hypertension) occurring after 20 weeks of gestation, combined with either new-onset proteinuria (excess protein in the urine) or other signs or symptoms involving multiple organ systems. The specific etiology of preeclampsia is unclear.2–6 Preeclampsia can lead to poor health outcomes in both the mother and infant. It is the second leading cause of maternal mortality worldwide 7,8 and may also lead to other serious maternal complications, including stroke, eclampsia, and organ failure. Adverse perinatal outcomes for the fetus and newborn include intrauterine growth restriction, low birth weight, and stillbirth. Many of the complications associated with preeclampsia lead to early induction of labor or cesarean delivery and subsequent preterm birth.
DETECTION
Obtaining blood pressure measurements to screen for preeclampsia could allow for early identification and diagnosis of the condition, resulting in close surveillance and effective treatment to prevent serious complications. The USPSTF has previously established that there is adequate evidence on the accuracy of blood pressure measurements to screen for preeclampsia.
The USPSTF found adequate evidence that testing for protein in the urine with a dipstick test has low diagnostic accuracy for detecting proteinuria in pregnancy.
BENEFITS OF EARLY DETECTION AND TREATMENT
Preeclampsia is a complex syndrome. It can quickly evolve into a severe disease that can result in serious, even fatal health outcomes for the mother and infant. The ability to screen for preeclampsia using blood pressure measurements is important to identify and effectively treat a potentially unpredictable and fatal condition. The USPSTF found adequate evidence that the well-established treatments of preeclampsia result in a substantial benefit for the mother and infant by reducing maternal and perinatal morbidity and mortality.
The USPSTF found inadequate evidence on the effectiveness of risk prediction tools (e.g., clinical indicators, serum markers, or uterine artery pulsatility index) that would support different screening strategies for predicting preeclampsia.
HARMS OF EARLY DETECTION AND TREATMENT
The USPSTF found adequate evidence to bound the potential harms of screening for and treatment of preeclampsia as no greater than small. This assessment was based on the known harms of treatment with antihypertension medications, induced labor, and magnesium sulfate; the likely few harms from screening with blood pressure measurements; and the potential poor maternal and perinatal outcomes resulting from severe untreated preeclampsia and eclampsia. The USPSTF found inadequate evidence on the harms of risk prediction.
USPSTF ASSESSMENT
The USPSTF concludes with moderate certainty that screening for preeclampsia in pregnant women with blood pressure measurements has a substantial net benefit.
Clinical Considerations
PATIENT POPULATION UNDER CONSIDERATION
This recommendation applies to pregnant women without a known diagnosis of preeclampsia or hypertension.
ASSESSMENT OF RISK
All pregnant women are at risk for preeclampsia and should be screened. Important clinical conditions associated with increased risk for preeclampsia include a history of eclampsia or preeclampsia (particularly early-onset preeclampsia), a previous adverse pregnancy outcome, maternal comorbid conditions (including type 1 or 2 diabetes prior to pregnancy, gestational diabetes, chronic hypertension, renal disease, and autoimmune diseases), and multifetal gestation.4,9 Other risk factors include nulliparity, obesity, African American race, low socioeconomic status, and advanced maternal age.4,9
In the United States, preeclampsia is more prevalent among African American women than among white women. Differences in prevalence may be, in part, due to African American women being disproportionately affected by risk factors for preeclampsia. African American women also have case fatality rates related to preeclampsia 3 times higher than rates among white women (73.5 vs. 27.4 per 100,000 cases).4,10–12 Higher prevalence and case fatality rates factor into why African American women are 3 times more likely to die of preeclampsia than white women.4,10–12 Inequalities in access to adequate prenatal care may contribute to poor outcomes associated with preeclampsia in African American women.4,12
SCREENING TESTS
Blood pressure measurements are routinely used as a screening tool for preeclampsia. The accuracy of blood pressure measurements has been well established.13 Sphygmomanometry is the recommended method for blood pressure measurement during pregnancy. The patient should be relaxed prior to measurement. After 5 minutes has elapsed, the patient's blood pressure should be read while she is in a sitting position, with her legs uncrossed and her back supported. The patient's arm should be at the level of the right atrium of the heart. If the patient's upper arm circumference is 33 cm or greater, a large blood pressure cuff should be used.5,13–15 Clinicians should avoid measuring blood pressure in the upper arm in the left lateral position because this position falsely lowers blood pressure readings.13–15
Recently revised criteria for the diagnosis of preeclampsia include elevated blood pressure (≥ 140/90 mm Hg on 2 occasions 4 hours apart, after 20 weeks of gestation) and either proteinuria (≥ 300 mg per dL on a 24-hour urine protein test, protein to creatinine ratio of ≥ 0.3 mg per mmol, or urine protein dipstick reading > 1 if quantitative analysis is not available) or, in the absence of proteinuria, thrombocytopenia, renal insufficiency, impaired liver function, pulmonary edema, or cerebral or visual symptoms.5
SCREENING INTERVAL
Blood pressure measurements should be obtained during each prenatal care visit throughout pregnancy. If a patient has an elevated blood pressure reading, the reading should be confirmed with repeated measurements. Further diagnostic evaluation and clinical monitoring are indicated for patients with elevated blood pressure on multiple measurements.
TREATMENT
ADDITIONAL APPROACHES TO PREVENTION
The USPSTF recommends the use of low-dose aspirin (81 mg per day) as preventive medication after 12 weeks of gestation in women who are at high risk for preeclampsia.9