
Am Fam Physician. 2018;97(11):729-734
Patient information: See related handout on excessive sweating (hyperhidrosis), written by the authors of this article.
Author disclosure: No relevant financial affiliations.
Hyperhidrosis is excessive sweating that affects patients' quality of life, resulting in social and work impairment and emotional distress. Primary hyperhidrosis is bilaterally symmetric, focal, excessive sweating of the axillae, palms, soles, or craniofacial region not caused by other underlying conditions. Secondary hyperhidrosis may be focal or generalized, and is caused by an underlying medical condition or medication use. The Hyperhidrosis Disease Severity Scale is a validated survey used to grade the tolerability of sweating and its impact on quality of life. The score can be used to guide treatment. Topical aluminum chloride solution is the initial treatment in most cases of primary focal hyperhidrosis. Topical glycopyrrolate is first-line treatment for craniofacial sweating. Botulinum toxin injection (onabotulinumtoxinA) is considered first- or second-line treatment for axillary, palmar, plantar, or craniofacial hyperhidrosis. Iontophoresis should be considered for treating hyperhidrosis of the palms and soles. Oral anticholinergics are useful adjuncts in severe cases of hyperhidrosis when other treatments fail. Local microwave therapy is a newer treatment option for axillary hyperhidrosis. Local surgery and endoscopic thoracic sympathectomy should be considered in severe cases of hyperhidrosis that have not responded to topical or medical therapies.
Hyperhidrosis is excessive sweating beyond what is physiologically required for thermoregulation, often causing social, emotional, and work impairment. This condition can be primary or secondary. Primary hyperhidrosis is idiopathic, bilaterally symmetric, excessive sweating of the axillae, palms, soles, face, and, less commonly, scalp or inguinal folds. Secondary hyperhidrosis may be focal or generalized, and is caused by an underlying medical condition or medication use.1,2
| Clinical recommendation | Evidence rating | References |
|---|---|---|
| The Hyperhidrosis Disease Severity Scale should be used to gauge severity of primary hyperhidrosis and predict response to treatment. | C | 2, 4, 6, 7 |
| Topical 20% aluminum chloride (Drysol) should be used as first-line treatment in most cases of primary hyperhidrosis, regardless of severity and location. | C | 2, 4 |
| Iontophoresis may be effective as first- or second-line treatment for primary hyperhidrosis of the palms or soles. | C | 2, 4 |
| Intradermal onabotulinumtoxinA (Botox) injections may be considered first- or second-line treatments for many cases of primary hyperhidrosis involving the axillae, palms, soles, or face. | C | 2, 4 |
| Oral anticholinergics are recommended if treatment with topical aluminum chloride, onabotulinumtoxinA injection, and iontophoresis is ineffective. | C | 4 |
| Local surgery and endoscopic thoracic sympathectomy should be considered only after topical and medical treatments have failed. | C | 2, 4, 9, 26, 29 |
Epidemiology
Hyperhidrosis affects 1% to 3% of the U.S. population, yet less than one-half of those affected discuss this with their physician.3 More than 90% of hyperhidrosis cases are primary, and more than one-half of these cases affect the axillae.2,3 More than one-third of persons with axillary hyperhidrosis report that the condition is barely tolerable or completely intolerable, and it nearly always interferes with daily activities.3 Up to two-thirds of patients report a family history, suggesting a genetic predisposition. Although prevalence between sexes is roughly equal, women are more likely to report hyperhidrosis to their physician.4
Etiology and Pathophysiology
The cause of primary hyperhidrosis is not well understood. Eccrine sweat glands—distributed throughout the body, but heavily concentrated on the palms, soles, axillae, and face—are innervated by postganglionic autonomic nerve fibers and stimulated by the neurotransmitter acetylcholine.4 It is thought that increased or aberrant sympathetic stimulation of the eccrine sweat glands is responsible for the increased sweating rather than an increased number or size of the glands.4 Persons with primary hyperhidrosis have a higher-than-normal basal level of sweat production and an increased response to normal stimuli, such as emotional or physical stress.
Diagnosis
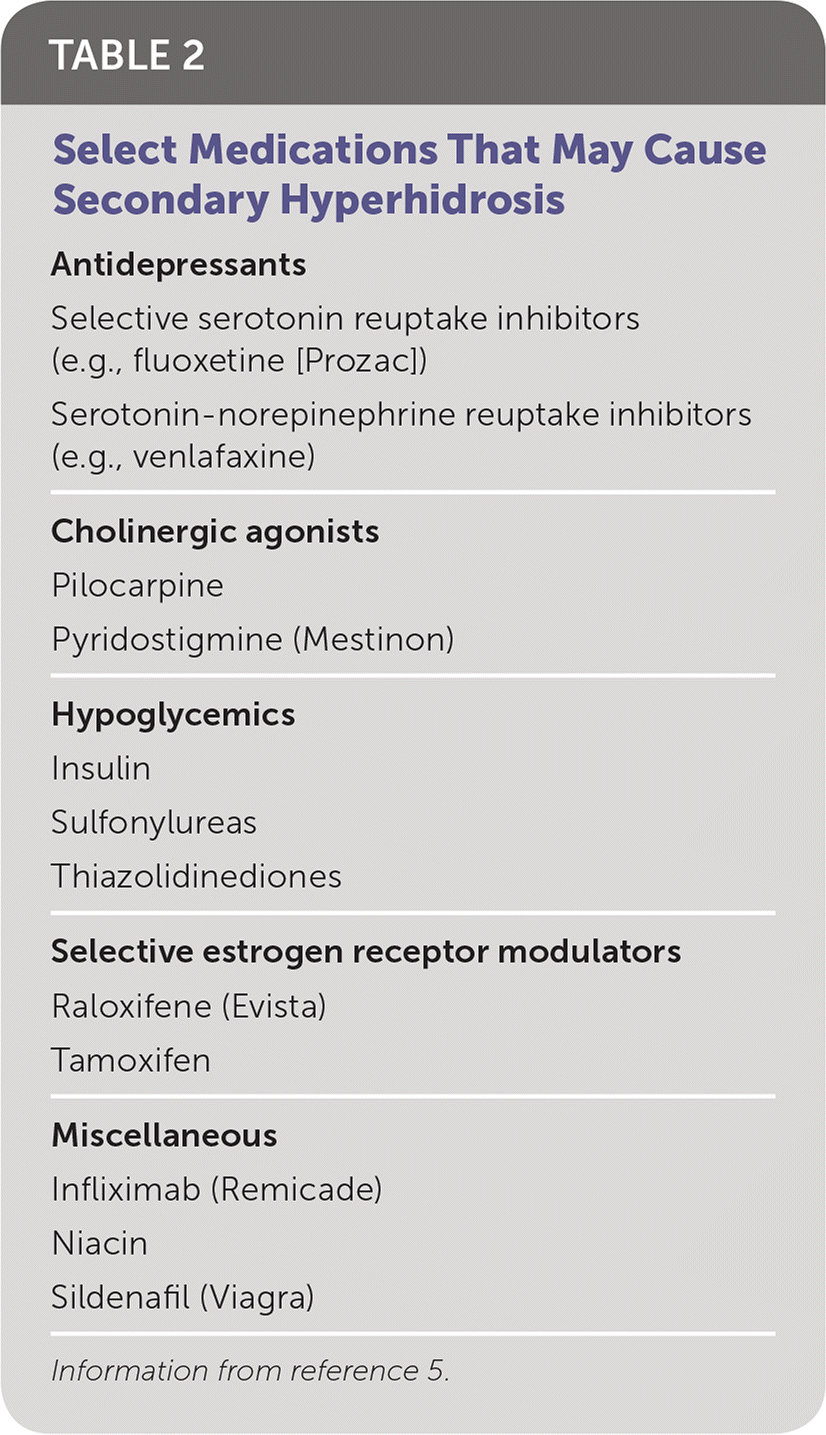
There are no controlled studies on the sensitivity and specificity of the history, physical examination, or testing to accurately diagnose primary hyperhidrosis or to quantify its severity. Criteria for diagnosis include focal, visible, and excessive sweating for longer than six months without apparent cause, and at least two of the following: bilateral and symmetric sweating, impairment of daily activities, occurrence at least once per week, age of onset younger than 25 years, no occurrence during sleep, and a positive family history.2 Requiring four criteria increases the discrimination between primary and secondary hyperhidrosis (positive predictive value = 0.99; negative predictive value = 0.85). Laboratory testing is not necessary unless history and physical examination suggest a secondary cause.2 There are several possible secondary causes for excessive sweating. Conditions and medications that can cause excessive sweating are listed in Table 11,2 and Table 2,5 respectively.

| Alcohol use |
| Chronic pulmonary disease; acute respiratory failure |
| Congestive heart failure |
| Endocrine/metabolic disorders (e.g., diabetes mellitus, thyrotoxicosis, hypoglycemia, hyperpituitarism) |
| Febrile illness/infection (e.g., defervescence, tuberculosis) |
| Gustatory (e.g., spicy foods) |
| Malignancies (e.g., carcinoid, pheochromocytoma) |
| Medications (Table 2) |
| Neurologic (e.g., Arnold-Chiari malformation, Parkinson disease, spinal cord injury) |
| Physiologic (e.g., menopause) |
| Psychiatric disease (e.g., generalized anxiety disorder, social anxiety) |
| Substance abuse; narcotic withdrawal |
| Antidepressants |
| Selective serotonin reuptake inhibitors (e.g., fluoxetine [Prozac]) |
| Serotonin-norepinephrine reuptake inhibitors (e.g., venlafaxine) |
| Cholinergic agonists |
| Pilocarpine |
| Pyridostigmine (Mestinon) |
| Hypoglycemics |
| Insulin |
| Sulfonylureas |
| Thiazolidinediones |
| Selective estrogen receptor modulators |
| Raloxifene (Evista) |
| Tamoxifen |
| Miscellaneous |
| Infliximab (Remicade) |
| Niacin |
| Sildenafil (Viagra) |
Hyperhidrosis negatively impacts daily life, especially emotional well-being, self-esteem, interpersonal relationships, and occupational productivity.6,7 Although several tools have been developed to measure the impact of hyperhidrosis on quality of life, most are too complex to incorporate into office practice. The Hyperhidrosis Disease Severity Scale (HDSS) is a validated single-question survey with four grades of tolerability of sweating and impact on quality of life.3 This survey can estimate the effect on daily activities and response to treatment.
The HDSS is scored as follows: 1 point for sweating that is not noticeable and does not interfere with daily activities; 2 points for sweating that is tolerable but sometimes interferes with daily activities; 3 points for sweating that is barely tolerable and often interferes with daily activities; and 4 points for intolerable sweating that always interferes with daily activities. A score of 2 is considered mild, whereas a score of 3 or 4 is considered severe.4
Treatment
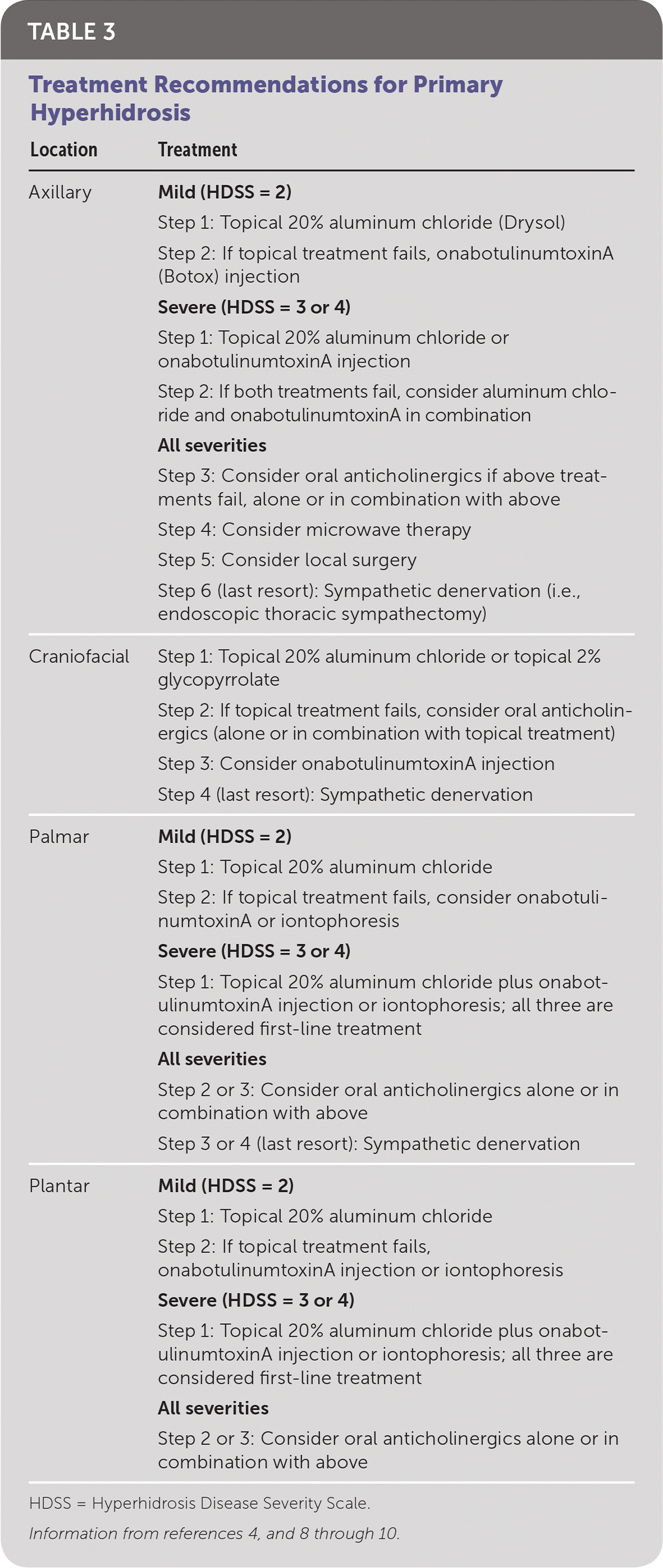
Disease severity should be measured using the HDSS.4 Treatment success is defined as a decrease in the HDSS score.4 Most treatment recommendations are based on expert consensus, because the evidence is poor (e.g., few study participants, nonrandomized trials, often not based on prospective trials). Table 3 summarizes the treatment options for hyperhidrosis based on location and severity.4,8–10
| Location | Treatment |
|---|---|
| Axillary | Mild (HDSS = 2) |
| Step 1: Topical 20% aluminum chloride (Drysol) | |
| Step 2: If topical treatment fails, onabotulinumtoxinA (Botox) injection | |
| Severe (HDSS = 3 or 4) | |
| Step 1: Topical 20% aluminum chloride or onabotulinumtoxinA injection | |
| Step 2: If both treatments fail, consider aluminum chloride and onabotulinumtoxinA in combination | |
| All severities | |
| Step 3: Consider oral anticholinergics if above treatments fail, alone or in combination with above | |
| Step 4: Consider microwave therapy | |
| Step 5: Consider local surgery | |
| Step 6 (last resort): Sympathetic denervation (i.e., endoscopic thoracic sympathectomy) | |
| Craniofacial | Step 1: Topical 20% aluminum chloride or topical 2% glycopyrrolate |
| Step 2: If topical treatment fails, consider oral anticholinergics (alone or in combination with topical treatment) | |
| Step 3: Consider onabotulinumtoxinA injection | |
| Step 4 (last resort): Sympathetic denervation | |
| Palmar | Mild (HDSS = 2) |
| Step 1: Topical 20% aluminum chloride | |
| Step 2: If topical treatment fails, consider onabotulinumtoxinA or iontophoresis | |
| Severe (HDSS = 3 or 4) | |
| Step 1: Topical 20% aluminum chloride plus onabotulinumtoxinA injection or iontophoresis; all three are considered first-line treatment | |
| All severities | |
| Step 2 or 3: Consider oral anticholinergics alone or in combination with above | |
| Step 3 or 4 (last resort): Sympathetic denervation | |
| Plantar | Mild (HDSS = 2) |
| Step 1: Topical 20% aluminum chloride | |
| Step 2: If topical treatment fails, onabotulinumtoxinA injection or iontophoresis | |
| Severe (HDSS = 3 or 4) | |
| Step 1: Topical 20% aluminum chloride plus onabotulinumtoxinA injection or iontophoresis; all three are considered first-line treatment | |
| All severities | |
| Step 2 or 3: Consider oral anticholinergics alone or in combination with above |
FIRST- AND SECOND-LINE THERAPIES
First-line treatment of all primary focal hyperhidrosis, regardless of severity, is topical 20% aluminum chloride (Drysol).2,4,11,12 This solution is applied nightly to the affected areas for six to eight hours until the HDSS score decreases, at which time the application interval can be lengthened to maintain sweat control.4,13 The aluminum salts cause an obstruction of the eccrine sweat glands and destruction of the secretory cells. This solution can result in skin irritation, but it can be diluted to decrease irritation if necessary. Use of over-the-counter “clinical strength” antiperspirants containing aluminum zirconium trichlorohydrate has shown a decrease in excessive sweating (as measured by sweat production, not the HDSS score), with less skin irritation than prescription-strength aluminum chloride solutions.14
For craniofacial hyperhidrosis, topical 2% glycopyrrolate (compounded by a pharmacy) may be considered first-line treatment. It has shown a 96% success rate (as measured by gravimetric chemical analysis and non-HDSS quality-of-life surveys) with minimal adverse effects (mild skin irritation), and can be applied once every two to three days.8
For palmar and plantar hyperhidrosis, iontophoresis may be effective as first- or second-line treatment.4 Iontophoresis is the passing of an ionized substance, usually water, through the skin by the application of a direct electrical current. Its mechanism of action is unknown.15,16 Tap water is poured into the device tray, and then the hands or feet are submerged while a direct electrical current is applied for a specified time, depending on the current. There are three devices registered with the U.S. Food and Drug Administration: RA Fischer MD-1a, RA Fischer MD-2, and Drionic. The procedure can be easily performed at home, and adverse effects (e.g., erythema, vesiculation, tingling) are typically mild and do not require cessation of the treatments.15,16 The procedure is performed three days per week until the desired effect is achieved, followed by a maintenance regimen of once per week. If tap water alone is not effective, adding a tablespoon of baking soda or one or two crushed tablets of the anticholinergic glycopyrrolate (Robinul) to each pan may be beneficial.16 A detailed description of a recommended application of the procedure is available in the literature.16
Botulinum toxin injection is the most studied hyperhidrosis treatment and demonstrates consistent improvement in HDSS scores and in sweat production as measured in the axillae and palms.4,9 It may be considered first- or second-line therapy for hyperhidrosis affecting the axillae, palms, soles, or face.2,4 Botulinum toxins bind synaptic proteins, blocking the release of acetylcholine from the cholinergic neurons that innervate the eccrine sweat glands.17 There are several commercially available botulinum toxin preparations approved by t he U.S. Food and Drug Administration that are available to physicians who are trained in this procedure. The most commonly used is onabotulinumtoxinA (Botox).17
OnabotulinumtoxinA is administered intradermally in the affected area. It is packaged as a 100-unit vial that is commonly divided into 50 units total for each side. The toxin is injected intradermally in 0.1 mL aliquots per cm2.17 It is important to determine the precise area to treat using the Minor starch-iodine test.17,18 For this test, a 3% to 5% iodine solution is first applied to the area to be treated and allowed to dry, and then starch is applied. The sweat turns purple when in contact with the iodine and starch, precisely identifying the areas to inject (see a video example of the Minor starch-iodine test and axillary injection at https://www.youtube.com/watch?v=U08PJhRQD0s).17,18 In most cases, treatment results last six to nine months.17,19,20 Adverse effects typically include injection-site pain and bruising, decreased grip strength when injected into the palms,17,19,20 and frontalis muscle weakness when used on the forehead.8
ALTERNATIVE THERAPIES AND PROCEDURES
Canadian guidelines recommend oral anticholinergics for treating primary hyperhidrosis with an HDSS score of 3 or 4 that does not resolve with topical aluminum chloride, onabotulinumtoxinA, or iontophoresis.4 The most commonly used oral anticholinergic medications are oxybutynin and glycopyrrolate.21 One study showed that oxybutynin, 2.5 to 10 mg per day, decreased excessive sweating and improved HDSS and non-HDSS quality-of-life scores (median = 76% of patients; range = 57% to 100%); however, 75% also experienced dry mouth.21 Although dry mouth is the most common adverse effect, patients may also experience abdominal symptoms, constipation, urinary retention, tachycardia, drowsiness, and blurred vision. On average, 10% of patients stop taking oxybutynin because of adverse effects.21 There is no evidence to quantify the benefit of glycopyrrolate; however, it also has a high prevalence (38%) of dry mouth.21
A newer, noninvasive local treatment of axillary hyperhidrosis uses microwave technology.22 The application of microwave energy destroys eccrine sweat glands by creating local heat, resulting in cellular thermolysis.22 This outpatient procedure is applied with a handheld transducer after mapping the axillae using the Minor starch-iodine test. Local anesthesia is required.22 This treatment results in a decrease in the HDSS score of at least one point in 94% of patients and at least two points in 55% of patients.23
Another emerging treatment in axillary hyperhidrosis is fractionated microneedle radiofrequency.24 During this procedure, microneedles are placed 2 to 3 mm under the skin, and radiofrequency energy is applied. This therapy results in a decrease in the HDSS score of at least one point in nearly 80% of patients.24,25
Local surgical therapy has been used to treat axillary hyperhidrosis. Techniques include radical surgical excision (rarely used because of high complication and recurrence rates), limited skin excision, liposuction, curettage, and liposuctioncurettage.26,27 Although these techniques can initially reduce measured axillary sweating, they have high relapse rates several months after the procedure.26,27
Because hyperhidrosis is thought to be secondary to excessive sympathetic stimulation, endoscopic thoracic sympathectomy has been used to treat severe cases of hyperhidrosis.28 This procedure, which has evolved from an open procedure to an endoscopic one, involves cutting or clipping sympathetic nerves.28 Referral for endoscopic thoracic sympathectomy may be indicated when less invasive therapies are ineffective.4,9,28 Although the procedure decreases or eliminates sweating in the original problem area, a common late complication is compensatory sweating in other areas, usually in the abdomen, back, gluteal region, and legs.29
Data Sources: The authors searched the Agency for Healthcare Research and Quality Evidence Reports, Cochrane Database of Systematic Reviews, National Guideline Clearinghouse, Essential Evidence Plus, and PubMed clinical queries using the following keywords: hyperhidrosis, excessive sweating, primary hyperhidrosis, secondary hyperhidrosis. Search date: September 24, 2017.
