
Am Fam Physician. 2019;99(5):314-323
See related AFP Community Blog post: Family Doctors Can Easily Treat Hepatitis B "In-House"
Patient information: See related handout on hepatitis B, written by the authors of this article.
Author disclosure: No relevant financial affiliations.
Hepatitis B virus (HBV) is a partly double-stranded DNA virus that causes acute and chronic liver infection. Screening for hepatitis B is recommended in pregnant women at their first prenatal visit and in adolescents and adults at high risk of chronic infection. Hepatitis B vaccination is recommended for medically stable infants weighing 2,000 g or more within 24 hours of birth, unvaccinated infants and children, and unvaccinated adults requesting protection from hepatitis B or who are at increased risk of infection. Acute hepatitis B is defined as the discrete onset of symptoms, the presence of jaundice or elevated serum alanine transaminase levels, and test results showing hepatitis B surface antigen and hepatitis B core antigen. There is no evidence that antiviral treatment is effective for acute hepatitis B. Chronic hepatitis B is defined as the persistence of hepatitis B surface antigen for more than six months. Individuals with chronic hepatitis B are at risk of hepatocellular carcinoma and cirrhosis, but morbidity and mortality are reduced with adequate treatment. Determining the stage of liver disease (e.g., evidence of inflammation, fibrosis) is important to guide therapeutic decisions and the need for surveillance for hepatocellular carcinoma. Treatment should be individualized based on clinical and laboratory characteristics and the risks of developing cirrhosis and hepatocellular carcinoma. Immunologic cure, defined as the loss of hepatitis B surface antigen with sustained HBV DNA suppression, is attainable with current drug therapies that suppress HBV DNA replication and improve liver inflammation and fibrosis. Pegylated interferon alfa-2a, entecavir, and tenofovir are recommended as first-line treatment options for chronic hepatitis B.
The Centers for Disease Control and Prevention (CDC) estimated that in 2015 there were 21,900 cases of acute hepatitis B, with an overall incidence of 1.1 cases per 100,000.1 There are an estimated 850,000 to 2.2 million individuals in the United States with chronic hepatitis B.1,2 Approximately 25% of children and 15% of adults with chronic hepatitis B die prematurely from hepatocellular carcinoma (HCC) or cirrhosis.3 However, treatment reduces morbidity and mortality from the disease.
WHAT IS NEW ON THIS TOPIC
Approximately 1,000 cases of perinatal hepatitis B occur annually in the United States, and nearly 90% of chronic hepatitis B cases in infants develop in the first year of life.
Hepatitis B vaccination is recommended for all medically stable infants weighing 2,000 g (4 lb, 6 oz) or more within 24 hours of birth, unvaccinated infants and children, and unvaccinated adults requesting protection from hepatitis B or who are at increased risk of hepatitis B.
| Clinical recommendation | Evidence rating | References |
|---|---|---|
| Pregnant women should be screened for hepatitis B at the first prenatal visit. | A | 6 |
| Adolescents and adults at high risk of chronic infection should be screened for hepatitis B. | B | 7 |
| Hepatitis B vaccination is recommended for all medically stable infants weighing 2,000 g (4 lb, 6 oz) or more within 24 hours of birth, unvaccinated infants and children, and unvaccinated adults requesting protection from hepatitis B or who are at increased risk. | C | 11 |
| Acute hepatitis B should not be treated with antivirals. | B | 17 |
| All infants born to mothers who are positive for hepatitis B surface antigen should receive hepatitis B immune globulin promptly and the hepatitis B vaccine by 24 hours of life. | C | 25 |
| Pegylated interferon alfa-2a (Pegasys), entecavir (Baraclude), and tenofovir are recommended as first-line treatment options for chronic hepatitis B. | C | 27 |
The hepatitis B virus (HBV) is a DNA virus that is unusual in that its genome is only partly double stranded. The host cell DNA polymerases repair the DNA into a covalently closed circular DNA.4 The accumulation of covalently closed circular DNA in the nucleus of the hepatocyte is the basis for the persistence of HBV despite antiviral therapy5 (eFigure A). The HBV has 10 genotypes (A through J) and more than 30 subtypes.4
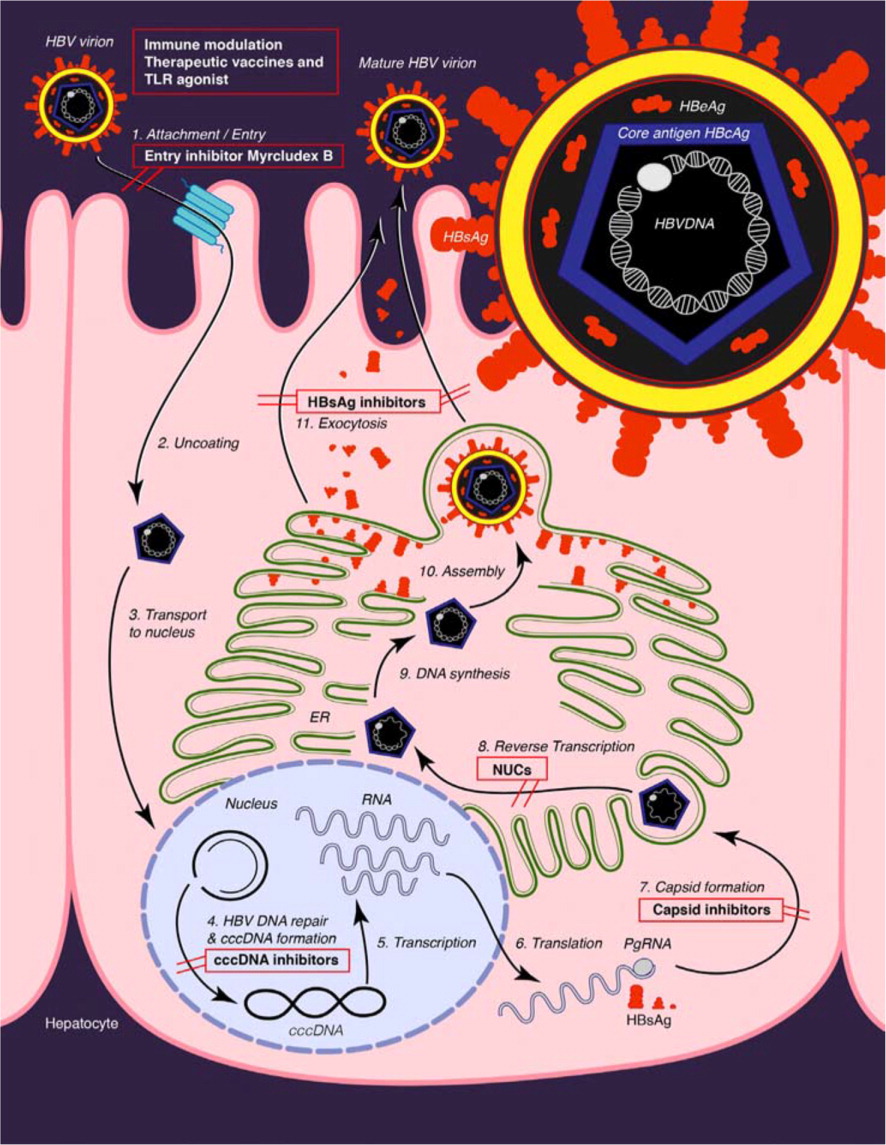
Screening and Prevention
The U.S. Preventive Services Task Force and American Academy of Family Physicians recommend screening for hepatitis B in pregnant women at the first prenatal visit and in adolescents and adults at high risk of chronic infection.6–8 In addition to other risk factors, the CDC uses a regional prevalence threshold of 2% or greater to define high risk.9 Table 1 includes indications for screening.7,9 Screening for hepatitis B includes testing for hepatitis B surface antigen (HBsAg) and, if positive, testing for antibodies to HBsAg (anti-HBs) and hepatitis B core antigen (anti-HBc) to distinguish between infection and immunity 7,10 (Table 211).

| The U.S. Preventive Services Task Force and CDC recommend screening in: Household contacts or sex partners of persons with hepatitis B Injection drug users Men who have sex with men Persons born in regions with ≥ 2% prevalence of chronic hepatitis B (e.g., Africa, Asia, Eastern Europe) Persons born in the United States who were not vaccinated as infants and whose parents are from regions with ≥ 8% prevalence of chronic hepatitis B Persons who are positive for HIV Pregnant women |
| The CDC additionally recommends screening in: Donors of blood, plasma, organs, tissue, or semen Infants born to mothers positive for hepatitis B surface antigen Persons on hemodialysis, cytotoxic therapy, or immunosuppressive therapy Persons who are sources of blood or bodily fluids that may expose others, requiring postexposure prophylaxis Persons with elevated alanine or aspartate transaminase levels of unknown etiology |
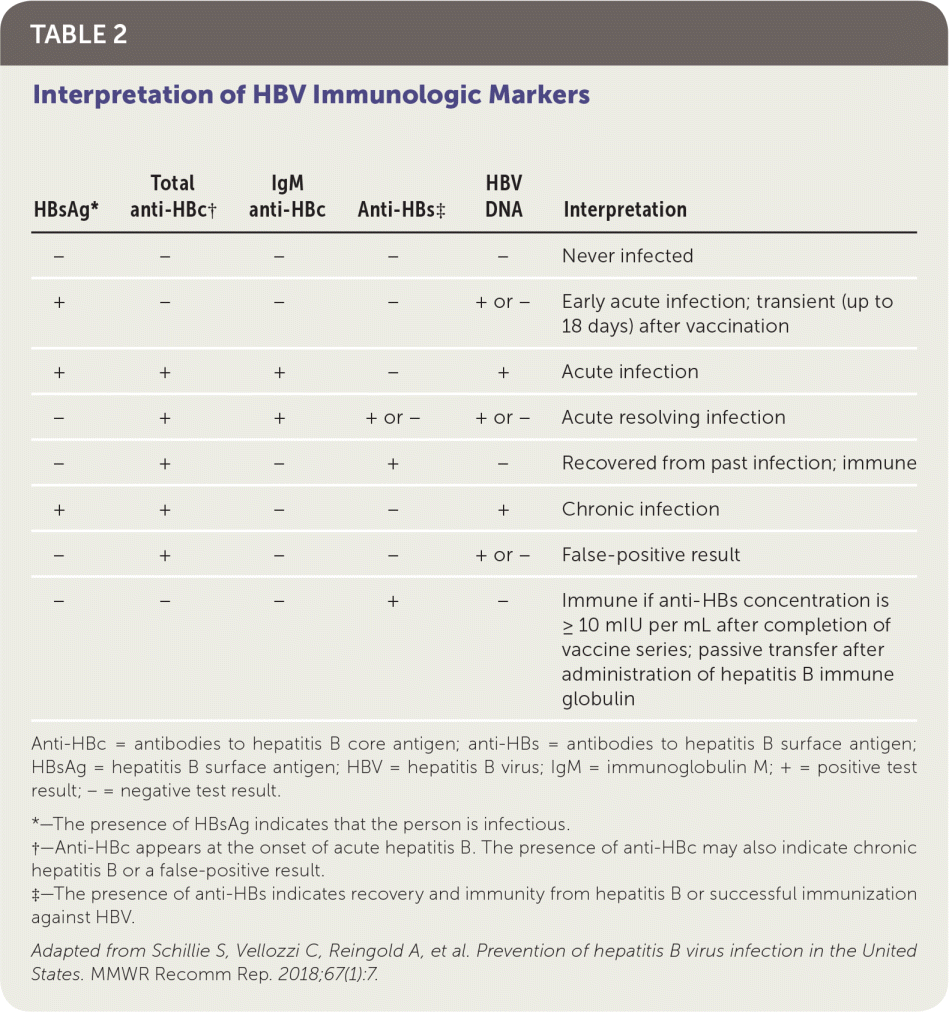
| HBsAg* | Total anti-HBc† | IgM anti-HBc | Anti-HBs‡ | HBV DNA | Interpretation |
|---|---|---|---|---|---|
| − | − | − | − | − | Never infected |
| + | − | − | − | + or − | Early acute infection; transient (up to 18 days) after vaccination |
| + | + | + | − | + | Acute infection |
| − | + | + | + or − | + or − | Acute resolving infection |
| − | + | − | + | − | Recovered from past infection; immune |
| + | + | − | − | + | Chronic infection |
| − | + | − | − | + or − | False-positive result |
| − | − | − | + | − | Immune if anti-HBs concentration is ≥ 10 mIU per mL after completion of vaccine series; passive transfer after administration of hepatitis B immune globulin |
IMMUNIZATION
The Advisory Committee on Immunization Practices recommends hepatitis B vaccination for all medically stable infants weighing 2,000 g (4 lb, 6 oz) or more within 24 hours of birth, unvaccinated infants and children, and unvaccinated adults requesting protection from hepatitis B or who are at increased risk of infection.11 There are several hepatitis B vaccines available, including the new two-dose vaccine, Heplisav-B.12,13
Postvaccination testing is recommended only in individuals who may not elicit a complete response to the vaccine based on risk factor assessment. In certain populations (i.e., persons on hemodialysis; persons who are immunocompromised, such as those with HIV infection; sex partners of persons positive for HBsAg; and health care personnel), testing for anti-HBs should be performed one to two months following the completion of the vaccine series.14,15 A responder is defined as a person with an anti-HBs level of 10 mIU per mL or more after completion of the vaccine series.3 If the anti-HBs level is less than 10 mIU per mL after the initial vaccine series, revaccination is indicated.15
Revaccination can be completed using one of two approaches: (1) administration of a second complete hepatitis B vaccine series followed by anti-HBs testing one to two months later, or (2) administration of a single dose of hepatitis B vaccine followed by anti-HBs testing one to two months later. If the anti-HBs level remains less than 10 mIU per mL after a single dose, completion of the series should be performed with anti-HBs testing one to two months after completing the series.12 A nonresponder is defined as a person with an anti-HBs level of less than 10 mIU per mL after six doses or more of the hepatitis B vaccine.3
Diagnosis
ACUTE HEPATITIS B
Acute hepatitis B is defined as the discrete onset of symptoms (e.g., fever, headache, malaise, anorexia, nausea, vomiting, diarrhea, abdominal pain), the presence of jaundice or elevated serum alanine transaminase (ALT) levels, and test results showing HBsAg and HBcAg.1 A Cochrane review of seven randomized controlled trials with 597 participants found that antiviral treatment has no benefit for acute hepatitis B based on low-quality or very low-quality evidence.17 An individual with acute hepatitis B may achieve complete immune clearance yielding lifelong immunity or develop chronic hepatitis B. The younger the age at the time of infection, the higher the probability of developing chronic infection.18
Hepatitis B e antigen (HBeAg) may be detected in the serum of individuals with early acute hepatitis B, soon after HBsAg becomes detectable. The presence of HBeAg in the serum correlates with high levels of infectivity. During recovery from acute hepatitis B, HBeAg becomes undetectable in the serum, while antibodies to HBeAg (anti-HBe) become detectable. Anti-HBe usually remain detectable for years after recovery.
CHRONIC HEPATITIS B
Chronic hepatitis B, defined as the persistence of HBsAg for more than six months, has five distinct phases19 (Table 320). The initial evaluation of individuals with chronic hepatitis B includes a complete history and examination. There should be a special emphasis on signs and symptoms of cirrhosis, evaluation of alcohol intake and metabolic risk factors, family history of HCC, and hepatitis A and B vaccination status.2
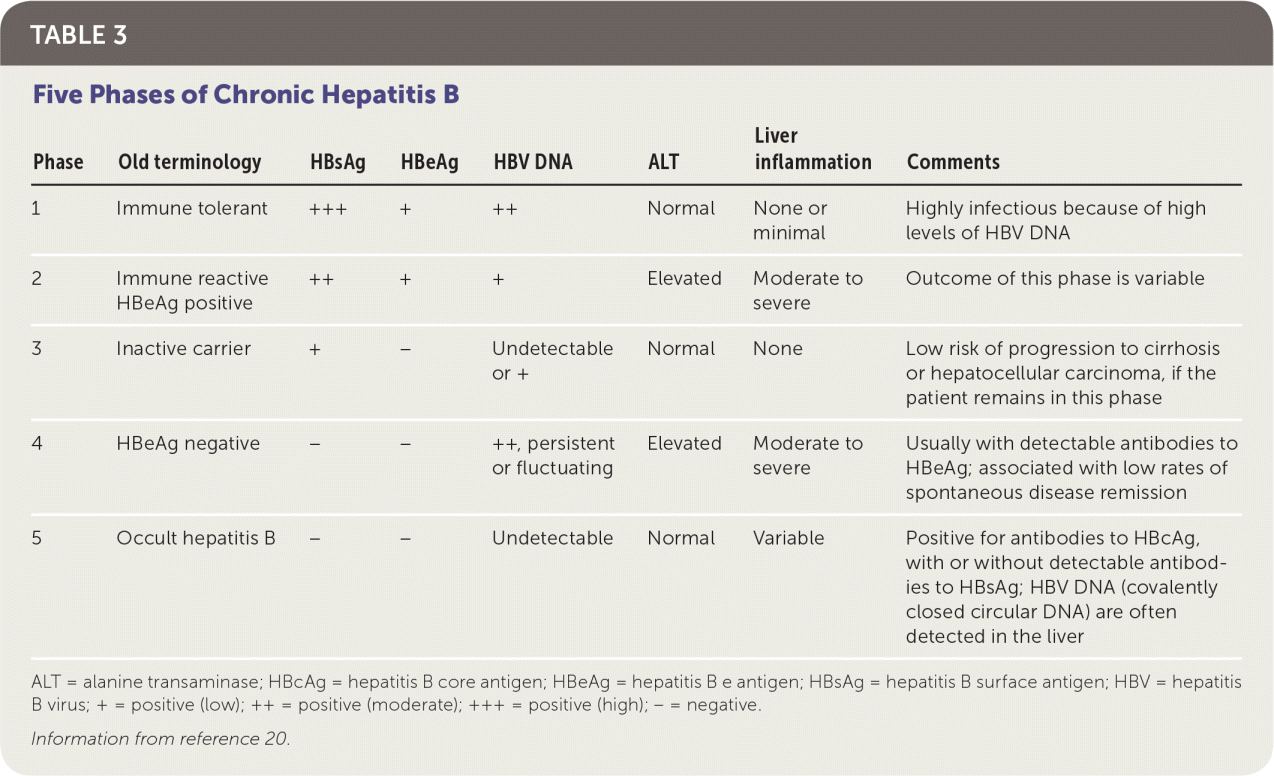
| Phase | Old terminology | HBsAg | HBeAg | HBV DNA | ALT | Liver inflammation | Comments |
|---|---|---|---|---|---|---|---|
| 1 | Immune tolerant | +++ | + | ++ | Normal | None or minimal | Highly infectious because of high levels of HBV DNA |
| 2 | Immune reactive HBeAg positive | ++ | + | + | Elevated | Moderate to severe | Outcome of this phase is variable |
| 3 | Inactive carrier | + | − | Undetectable or + | Normal | None | Low risk of progression to cirrhosis or hepatocellular carcinoma, if the patient remains in this phase |
| 4 | HBeAg negative | − | − | ++, persistent or fluctuating | Elevated | Moderate to severe | Usually with detectable antibodies to HBeAg; associated with low rates of spontaneous disease remission |
| 5 | Occult hepatitis B | − | − | Undetectable | Normal | Variable | Positive for antibodies to HBcAg, with or without detectable antibodies to HBsAg; HBV DNA (covalently closed circular DNA) are often detected in the liver |
Laboratory measurements include a complete blood count with platelets, aspartate transaminase, ALT, total bilirubin, alkaline phosphatase, albumin, and international normalized ratio. Serology testing includes HBeAg, anti-HBe, HBV DNA quantitation or viral load, HBV genotype, and anti–hepatitis A virus to determine the need for vaccination.2 Testing for coinfection with hepatitis C virus and HIV is recommended.2
The resolution of chronic hepatitis B is defined as the clearance of HBsAg with the detection of anti-HBs. Annually, approximately 0.5% of individuals with inactive chronic hepatitis B will have spontaneous clearance of HBsAg, and most will develop anti-HBs.2 Among adults with untreated chronic hepatitis B, the cumulative five-year incidence of cirrhosis is 8% to 20%, and the risk of HCC is 2% to 5%.2
The risk of liver-related complications is variable and influenced by a variety of host, viral, and environmental factors.2 Determining the stage of liver disease (e.g., evidence of inflammation, fibrosis) is important to guide therapeutic decisions and the need for HCC screening.2 Although liver biopsy is recommended for assessing inflammatory activity and fibrosis, noninvasive tests, such as transient elastography or a serum fibrosis panel, are also useful.2
It is unclear which patients might benefit from screening for HCC. Some experts recommend screening patients with chronic hepatitis B only if they have other risk factors for HCC, whereas others advocate screening all individuals with chronic hepatitis B.
A Cochrane review of three randomized controlled trials found insufficient evidence to support or refute the value of alpha fetoprotein testing, ultrasound screening, or both.21 However, a randomized controlled trial of individuals with chronic hepatitis B found a 37% reduction in mortality for those who underwent surveillance vs. those who did not.22
Chronic hepatitis B accounts for approximately one-half of all HCC cases.23 Recent guidelines recommend screening for HCC every six months with abdominal ultrasonography and alpha fetoprotein testing.24 If ultrasound findings are abnormal, then computed tomography or magnetic resonance imaging of the liver is recommended.23 Treatment does not eliminate the risk of HCC; therefore, surveillance for HCC should continue.2
Postexposure Management
OCCUPATIONAL EXPOSURE
The CDC provides detailed guidance on the management of health care personnel potentially exposed to the HBV.3 All health care personnel should immediately report any blood or bodily fluid exposures to their occupational health offices. After exposure to blood or bodily fluids, the patient's hepatitis B vaccination status should be assessed to determine if he or she is a known responder to the vaccine based on an anti-HBs level of 10 mIU per mL or more. Informed consent should be obtained from the source person in accordance with state laws, and his or her blood should be obtained and tested for HBsAg. If it is not possible to test the source person's blood, the exposed patient should be managed as if the source person is positive for HBsAg.
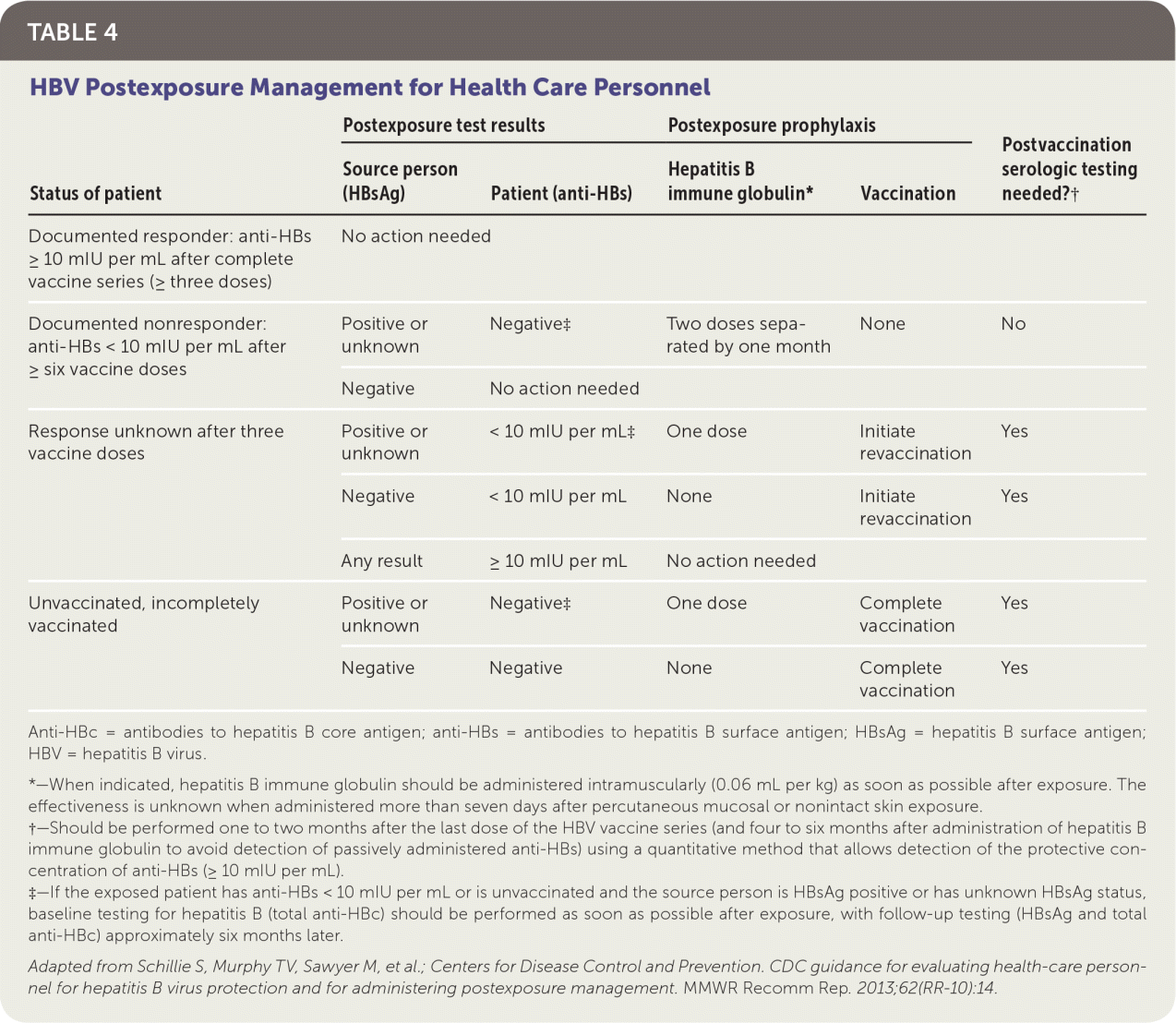
| Status of patient | Postexposure test results | Postexposure prophylaxis | Postvaccination serologic testing needed?† | ||
|---|---|---|---|---|---|
| Source person (HBsAg) | Patient (anti-HBs) | Hepatitis B immune globulin* | Vaccination | ||
| Documented responder: anti-HBs ≥ 10 mIU per mL after complete vaccine series (≥ three doses) | No action needed | ||||
| Documented nonresponder: anti-HBs < 10 mIU per mL after ≥ six vaccine doses | Positive or unknown | Negative‡ | Two doses separated by one month | None | No |
| Negative | No action needed | ||||
| Response unknown after three vaccine doses | Positive or unknown | < 10 mIU per mL‡ | One dose | Initiate revaccination | Yes |
| Negative | < 10 mIU per mL | None | Initiate revaccination | Yes | |
| Any result | ≥ 10 mIU per mL | No action needed | |||
| Unvaccinated, incompletely vaccinated | Positive or unknown | Negative‡ | One dose | Complete vaccination | Yes |
| Negative | Negative | None | Complete vaccination | Yes | |
PERINATAL EXPOSURE
Approximately 1,000 cases of perinatal hepatitis B occur annually in the United States, and nearly 90% of chronic hepatitis B in infants develops in the first year of life.25 All infants born to mothers who are HBsAg positive should receive hepatitis B immune globulin promptly and the hepatitis B vaccine by 24 hours of life.25 The vaccination series should be completed, and postvaccination serologic testing should be performed at nine to 12 months of age to assess response to vaccination. Additional vaccine doses should be administered if the infant is determined to be a nonresponder.26
Treatment
GOALS OF THERAPY
Immunologic cure, defined as the loss of HBsAg with sustained HBV DNA suppression, is attainable with current drug therapies. However, because current treatments cannot eradicate the virus, including the covalently closed circular DNA, reactivation may occur.2 Goals of therapy that correlate with improvements in patient-oriented outcomes include HBV DNA suppression, HBeAg loss/seroconversion (for individuals who were HBeAg positive), ALT normalization, and HBsAg loss.2
TREATMENT INDICATIONS
Lifelong monitoring is required for individuals with chronic hepatitis B who are not currently candidates for treatment, because they may become candidates in the future.27 For these patients, ALT levels should be monitored every three months during the first year and every six to 12 months thereafter. If ALT levels or aspartate transaminase levels become elevated, HBV DNA testing should be performed, and ALT levels should be monitored more often.2
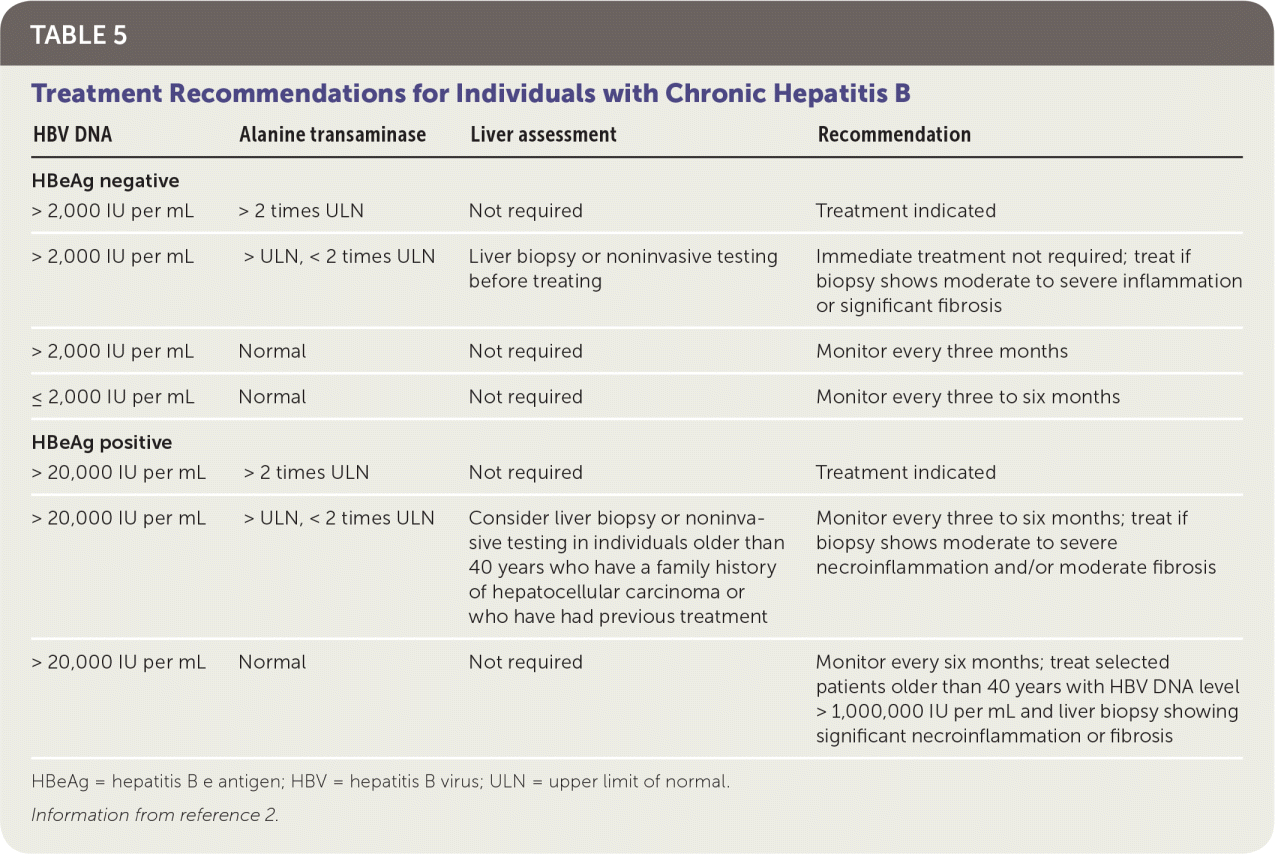
| HBV DNA | Alanine transaminase | Liver assessment | Recommendation |
|---|---|---|---|
| HBeAg negative | |||
| > 2,000 IU per mL | > 2 times ULN | Not required | Treatment indicated |
| > 2,000 IU per mL | > ULN, < 2 times ULN | Liver biopsy or noninvasive testing before treating | Immediate treatment not required; treat if biopsy shows moderate to severe inflammation or significant fibrosis |
| > 2,000 IU per mL | Normal | Not required | Monitor every three months |
| ≤ 2,000 IU per mL | Normal | Not required | Monitor every three to six months |
| HBeAg positive | |||
| > 20,000 IU per mL | > 2 times ULN | Not required | Treatment indicated |
| > 20,000 IU per mL | > ULN, < 2 times ULN | Consider liver biopsy or noninvasive testing in individuals older than 40 years who have a family history of hepatocellular carcinoma or who have had previous treatment | Monitor every three to six months; treat if biopsy shows moderate to severe necroinflammation and/or moderate fibrosis |
| > 20,000 IU per mL | Normal | Not required | Monitor every six months; treat selected patients older than 40 years with HBV DNA level > 1,000,000 IU per mL and liver biopsy showing significant necroinflammation or fibrosis |
TREATMENT OPTIONS
There are eight approved treatments for chronic hepatitis B in the United States.2 These antiviral treatments fall into two classes: peginterferon alfa-2a agents and nucleoside/nucleotide analogues2 (Table 62,28–36). Pegylated interferon alfa-2a (Pegasys), entecavir (Baraclude), and tenofovir are recommended as first-line treatment options.27 There are two treatment approaches: a defined treatment period with a peginterferon alfa-2a or long-term treatment with a nucleoside/nucleotide analogue.
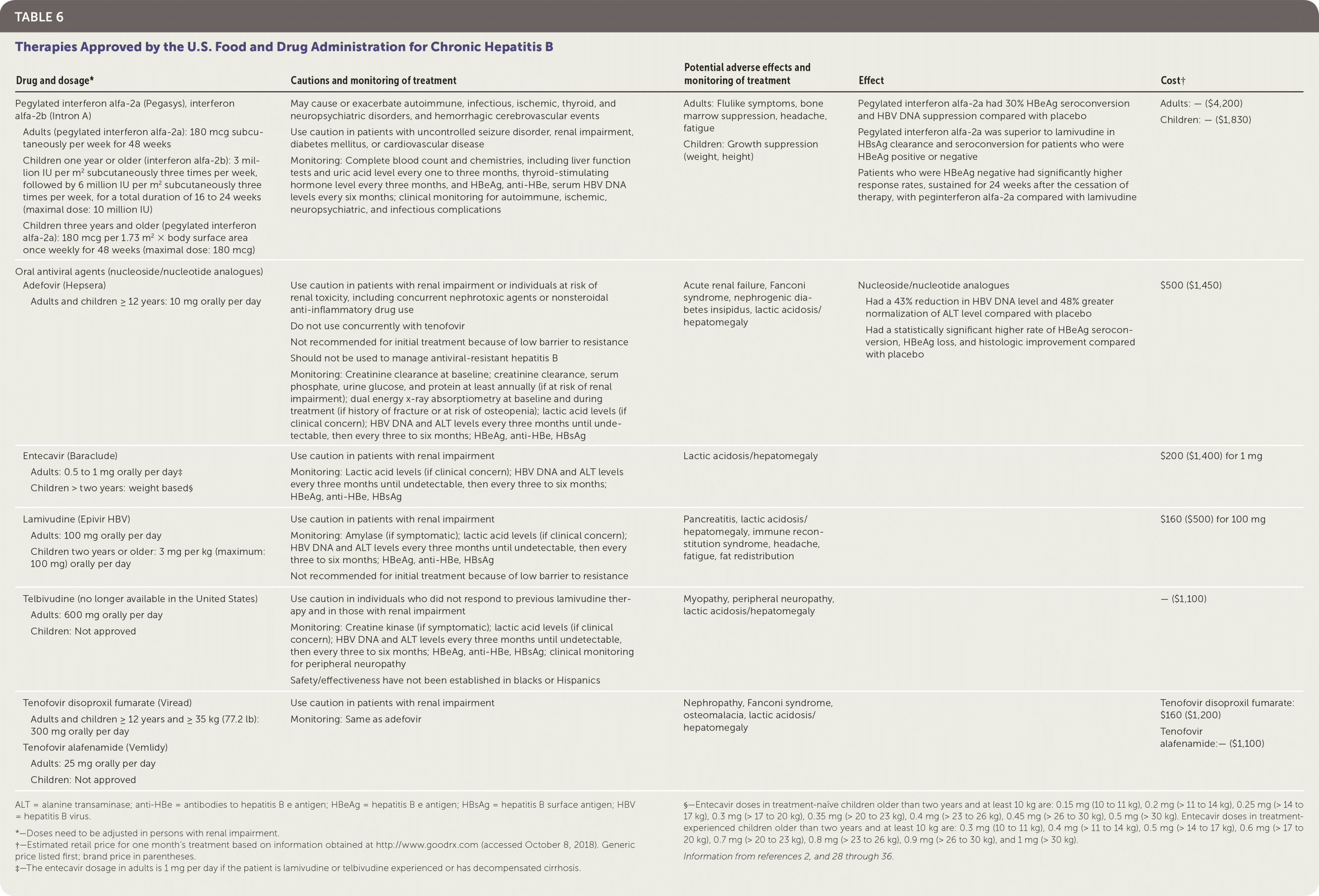
| Drug and dosage* | Cautions and monitoring of treatment | Potential adverse effects and monitoring of treatment | Effect | Cost† |
|---|---|---|---|---|
| Pegylated interferon alfa-2a (Pegasys), interferon alfa-2b (Intron A) Adults (pegylated interferon alfa-2a): 180 mcg subcutaneously per week for 48 weeks Children one year or older (interferon alfa-2b): 3 million IU per m2 subcutaneously three times per week, followed by 6 million IU per m2 subcutaneously three times per week, for a total duration of 16 to 24 weeks (maximal dose: 10 million IU) Children three years and older (pegylated interferon alfa-2a): 180 mcg per 1.73 m2 × body surface area once weekly for 48 weeks (maximal dose: 180 mcg) | May cause or exacerbate autoimmune, infectious, ischemic, thyroid, and neuropsychiatric disorders, and hemorrhagic cerebrovascular events Use caution in patients with uncontrolled seizure disorder, renal impairment, diabetes mellitus, or cardiovascular disease Monitoring: Complete blood count and chemistries, including liver function tests and uric acid level every one to three months, thyroid-stimulating hormone level every three months, and HBeAg, anti-HBe, serum HBV DNA levels every six months; clinical monitoring for autoimmune, ischemic, neuropsychiatric, and infectious complications | Adults: Flulike symptoms, bone marrow suppression, headache, fatigue Children: Growth suppression (weight, height) | Pegylated interferon alfa-2a had 30% HBeAg seroconversion and HBV DNA suppression compared with placebo Pegylated interferon alfa-2a was superior to lamivudine in HBsAg clearance and seroconversion for patients who were HBeAg positive or negative Patients who were HBeAg negative had significantly higher response rates, sustained for 24 weeks after the cessation of therapy, with peginterferon alfa-2a compared with lamivudine | Adults: — ($4,200) Children: — ($1,830) |
| Oral antiviral agents (nucleoside/nucleotide analogues) Adefovir (Hepsera) Adults and children ≥ 12 years: 10 mg orally per day | Use caution in patients with renal impairment or individuals at risk of renal toxicity, including concurrent nephrotoxic agents or nonsteroidal anti-inflammatory drug use Do not use concurrently with tenofovir Not recommended for initial treatment because of low barrier to resistance Should not be used to manage antiviral-resistant hepatitis B Monitoring: Creatinine clearance at baseline; creatinine clearance, serum phosphate, urine glucose, and protein at least annually (if at risk of renal impairment); dual energy x-ray absorptiometry at baseline and during treatment (if history of fracture or at risk of osteopenia); lactic acid levels (if clinical concern); HBV DNA and ALT levels every three months until undetectable, then every three to six months; HBeAg, anti-HBe, HBsAg | Acute renal failure, Fanconi syndrome, nephrogenic diabetes insipidus, lactic acidosis/hepatomegaly | Nucleoside/nucleotide analogues Had a 43% reduction in HBV DNA level and 48% greater normalization of ALT level compared with placebo Had a statistically significant higher rate of HBeAg seroconversion, HBeAg loss, and histologic improvement compared with placebo | $500 ($1,450) |
| Entecavir (Baraclude) Adults: 0.5 to 1 mg orally per day‡ Children > two years: weight based§ | Use caution in patients with renal impairment Monitoring: Lactic acid levels (if clinical concern); HBV DNA and ALT levels every three months until undetectable, then every three to six months; HBeAg, anti-HBe, HBsAg | Lactic acidosis/hepatomegaly | $200 ($1,400) for 1 mg | |
| Lamivudine (Epivir HBV) Adults: 100 mg orally per day Children two years or older: 3 mg per kg (maximum: 100 mg) orally per day | Use caution in patients with renal impairment Monitoring: Amylase (if symptomatic); lactic acid levels (if clinical concern); HBV DNA and ALT levels every three months until undetectable, then every three to six months; HBeAg, anti-HBe, HBsAg Not recommended for initial treatment because of low barrier to resistance | Pancreatitis, lactic acidosis/hepatomegaly, immune reconstitution syndrome, headache, fatigue, fat redistribution | $160 ($500) for 100 mg | |
| Telbivudine (no longer available in the United States) Adults: 600 mg orally per day Children: Not approved | Use caution in individuals who did not respond to previous lamivudine therapy and in those with renal impairment Monitoring: Creatine kinase (if symptomatic); lactic acid levels (if clinical concern); HBV DNA and ALT levels every three months until undetectable, then every three to six months; HBeAg, anti-HBe, HBsAg; clinical monitoring for peripheral neuropathy Safety/effectiveness have not been established in blacks or Hispanics | Myopathy, peripheral neuropathy, lactic acidosis/hepatomegaly | — ($1,100) | |
| Tenofovir disoproxil fumarate (Viread) Adults and children ≥ 12 years and ≥ 35 kg (77.2 lb): 300 mg orally per day Tenofovir alafenamide (Vemlidy) Adults: 25 mg orally per day Children: Not approved | Use caution in patients with renal impairment Monitoring: Same as adefovir | Nephropathy, Fanconi syndrome, osteomalacia, lactic acidosis/hepatomegaly | Tenofovir disoproxil fumarate: $160 ($1,200) Tenofovir alafenamide:— ($1,100) |
There are clear advantages and disadvantages to each approach. A meta-analysis including 14 studies involving 2,829 individuals found improved treatment effectiveness with combined therapy (peginterferon alfa-2a and nucleoside/nucleotide analogue); however, because of heterogeneity among trials and lack of consistent evidence, current guidelines recommend monotherapy.28
Subcutaneous Pegylated Interferon Alfa-2a. Pegylated interferon alfa-2a is administered for 48 weeks and should be considered in individuals with predictors of favorable treatment response and who may benefit from a defined treatment duration (e.g., low pretreatment HBV DNA and high ALT levels, HBV genotypes A or B, young women who wish to become pregnant in the future, concomitant hepatitis C, and younger age).27,28 Pegylated interferon alfa-2a has shown slightly higher seroconversion rates than nucleoside/nucleotide analogues; however, treatment outcomes differ among HBV genotypes.
The primary drawback of peginterferon alfa-2a is tolerability because this therapy is associated with frequent adverse effects (i.e., flulike symptoms, fatigue, mood disturbances, cytopenias, autoimmune disorders in adults, and anorexia and weight loss in children).2,29 The safety and effectiveness of peginterferon alfa-2a has been demonstrated in several studies conducted in individuals with HBeAg-positive and HBeAg-negative chronic hepatitis B.30–33
Oral Antiviral Agents. The nucleoside/nucleotide analogues approved in the United States for treatment of chronic hepatitis B are adefovir (Hepsera), entecavir, lamivudine (Epivir HBV), telbivudine (no longer available in the United States), tenofovir disoproxil fumarate (Viread), and tenofovir alafenamide (Vemlidy). These drugs, which target HBV by inhibiting the viral polymerase, are the most commonly used antivirals for treating chronic hepatitis B. They have excellent tolerability and safety profiles; however, the duration of their use is often indefinite because of frequent relapses or reactivation of hepatitis B after cessation of treatment.29 Entecavir, tenofovir, and tenofovir alafenamide are preferred because of their higher antiviral potency and lower resistance rates.27
The 2018 guidelines from the American Association for the Study of Liver Diseases recommend tenofovir alafenamide for initial therapy in adults with immune-active chronic hepatitis B. Tenofovir alafenamide should also be considered in patients with or at risk of renal dysfunction or bone disease. However, it is not recommended for patients who have a creatinine clearance less than 15 mL per minute per 1.73 m2 (0.25 mL per second per m2) or who are on dialysis.37 Multiple randomized controlled trials have shown excellent tolerability and effectiveness when comparing entecavir, tenofovir, and tenofovir alafenamide with other nucleoside/nucleotide analogues.38–42
PREGNANCY
Treating pregnant women who are HBsAg positive reduces perinatal transmission rates. Tenofovir is the preferred antiviral in pregnant women because it has a better resistance profile and there are more safety data in pregnant women with hepatitis B.43 The CDC recommends testing HBsAg-positive pregnant women for HBV-DNA to identify infants at greatest risk of perinatal HBV transmission and to guide maternal antiviral therapy.11
Decompensated Cirrhosis/Liver Transplant
Individuals with decompensated cirrhosis and chronic hepatitis B should be treated with a nucleoside/nucleotide analogue and assessed for liver transplantation eligibility.19 There is strong evidence that antiviral therapy improves liver function, increases survival, and avoids the need for liver transplantation when anti-HBV treatment is initiated early in decompensated cirrhosis.19
After liver transplantation to prevent recurrence of hepatitis B, low-risk patients may be treated with nucleoside/nucleotide analogue monotherapy, with or without hepatitis B immune globulin, and high-risk patients should be treated with both hepatitis B immune globulin and a nucleoside/nucleotide analogue.19
This article updates previous articles on this topic by Wilkins, et al.,20 and Lin and Kirchner.44
Data sources: We completed a general PubMed search using the MeSH term hepatitis B and excluding the MeSH terms hepatitis C and hepatitis D. The term hepatitis B was also used in a number of specialized searches looking into specific topics in combination with one or more of the following terms: child, pediatric, adult, caregivers, liver disease, liver cancer, treatment, vaccinations, screenings. The search included meta-analyses, randomized-controlled trials, and practice guidelines within the past 20 years. Also searched were the Cochrane database and Essential Evidence Plus. Search dates: October 2017 and November 2018.
