
Am Fam Physician. 2020;102(4):234-240
Related Putting Prevention into Practice: Primary Care Interventions for Prevention and Cessation of Tobacco Use in Children and Adolescents
As published by the USPSTF.
Summary of Recommendations and Evidence
The USPSTF recommends that primary care clinicians provide interventions, including education or brief counseling, to prevent initiation of tobacco use among school-aged children and adolescents (Table 1). B recommendation.

| What does the USPSTF recommend? | School-aged children and adolescents who have not started to use tobacco: Grade B Provide interventions, including education or brief counseling, to prevent initiation of tobacco use. |
| School-aged children and adolescents who use tobacco: I statement The evidence is insufficient to assess the balance of benefits and harms of primary care–feasible interventions for cessation of tobacco use. | |
| To whom does this recommendation apply? | School-aged children and adolescents younger than 18 years. |
| What's new? | This recommendation is consistent with the 2013 USPSTF recommendation, with some key updates: Adds a new I statement on interventions for the cessation of tobacco use in school-aged children and adolescents. Includes e-cigarettes as a tobacco product. |
| How to implement this recommendation? | Definition of tobacco use: Tobacco use refers to any tobacco product, including cigarettes, cigars (including cigarillos and little cigars), as well as vaping e-cigarettes.
|
| What are other relevant USPSTF recommendations? | The USPSTF has made recommendations on behavioral and pharmacotherapy interventions for tobacco smoking cessation in adults, including pregnant women, and primary care behavioral interventions to reduce illicit drug and nonmedical pharmaceutical use in children and adolescents. These recommendations are available at www.uspreventiveservicestaskforce.org. |
| Where to read the full recommendation statement? | Visit the USPSTF website (www.uspreventiveservicestaskforce.org) to read the full recommendation statement. This includes more details on the rationale of the recommendation, including benefits and harms; supporting evidence; and recommendations of others. |
The USPSTF concludes that the current evidence is insufficient to assess the balance of benefits and harms of primary care–feasible interventions for the cessation of tobacco use among school-aged children and adolescents (Table 1). I statement.
See the Practice Considerations section for more information on effective interventions to prevent initiation of tobacco use and for suggestions for practice regarding the I statement.
Importance
Tobacco use is the leading cause of preventable death in the United States.1 An estimated annual 480,000 deaths are attributable to tobacco use in adults, including secondhand smoke.1 It is estimated that every day about 1600 youth aged 12 to 17 years smoke their first cigarette2 and that about 5.6 million adolescents alive today will die prematurely of a smoking-related illness.1,3 Although conventional cigarette use has gradually declined among children in the United States since the late 1990s,4 tobacco use via electronic cigarettes (e-cigarettes) is quickly rising and is now more common among youth than cigarette smoking. e-Cigarette sales in the U.S. market have risen rapidly since 2007,5 and e-cigarette use by youth has been tracked in the National Youth Tobacco Survey since 2011.1 From 2011 to 2019, current e-cigarette use increased from 1.5% to 27.5% among high school students6,7 (from an estimated 220,000 to 4.11 million students); in 2019, 5.8% of high school students (an estimated 860,000 students) used conventional cigarettes.7
e-Cigarette products usually contain nicotine,8 which is addictive, raising concerns about e-cigarette use and nicotine addiction in children.5 Evidence suggests an association between e-cigarette use in nonsmoking adolescents and subsequent cigarette smoking in young adults. Ever use of e-cigarettes is associated with increased risk of ever use of combustible tobacco products.9 In addition, as the degree of e-cigarette use increases, frequency and intensity of smoking cigarettes also increase.9 Exposure to nicotine during adolescence can harm the developing brain, which may affect brain function and cognition, attention, and mood1,5,10,11; thus, minimizing nicotine exposure from any tobacco product in youth is important. In 2019, an outbreak of e-cigarette, or vaping, product use–associated lung injury (EVALI) occurred in the United States; approximately 15% of patients hospitalized with EVALI were younger than 18 years.12 Vitamin E acetate, an additive to some tetrahydrocannabinol-containing e-cigarettes, was found to be strongly linked to the outbreak.12 Other tobacco products high school students report using include cigars, cigarillos, and little cigars (7.6%); smokeless tobacco (4.8%); hookahs (3.4%); and pipe tobacco (1.1%).7 In 2019, cigar use (including cigarillos and little cigars) surpassed cigarette use in high school students.7 See the Definitions section for more information on tobacco products and terminology used in this USPSTF recommendation.
USPSTF Assessment of Magnitude of Net Benefit
Available evidence on interventions to prevent and help youth quit tobacco use almost exclusively focuses on cigarette smoking. For this recommendation, the USPSTF found this evidence to be most applicable to smoking combustible products (including cigarettes, cigars, cigarillos, and little cigars) and use of e-cigarettes (vaping).
See Table 2 for more information on the USPSTF recommendation rationale and assessment. For more details on the methods the USPSTF uses to determine the net benefit, see the USPSTF Procedure Manual.13 For a summary of the evidence that served as the basis for the recommendations, see the review of the evidence on the benefits and harms of primary care interventions for tobacco use prevention and cessation in children and adolescents.14,15

| Rationale | Prevention | Cessation |
|---|---|---|
| Benefits of intervention | Adequate evidence that behavioral counseling interventions can have a moderate effect in preventing initiation of tobacco use in school-aged children and adolescents | Inadequate evidence on behavioral counseling interventions for cessation of tobacco use in school-aged children and adolescents because many studies had small sample sizes and may not have been adequately powered to detect a benefit Inadequate evidence on medications for cessation of tobacco use in school-aged children and adolescents because of an inadequate number of studies |
| Harms of intervention | Adequate evidence to bound harms of behavioral counseling interventions as no greater than small based on the absence of reported harms in the evidence, the noninvasive nature of the interventions, and the low likelihood of serious harms | Adequate evidence to bound harms of behavioral counseling interventions as no greater than small based on the absence of reported harms in the evidence, the noninvasive nature of the interventions, and the low likelihood of serious harms Inadequate evidence on harms of medications |
| USPSTF assessment | Moderate certainty that primary care–relevant behavioral interventions to prevent tobacco use in school-aged children and adolescents have a moderate net benefit | Insufficient evidence to determine the balance of benefits and harms of primary care interventions for tobacco cessation in school-aged children and adolescents who already smoke |
PREVENTION
The USPSTF concludes with moderate certainty that primary care–feasible behavioral interventions, including education or brief counseling, to prevent tobacco use in school-aged children and adolescents have a moderate net benefit. The USPSTF found adequate evidence that behavioral counseling interventions, such as face-to-face or telephone interaction with a health care clinician, print materials, and computer applications, can have a moderate effect in preventing initiation of tobacco use in school-aged children and adolescents. The USPSTF sought but found no evidence on the harms of behavioral counseling interventions for the prevention or cessation of tobacco use; however, the USPSTF bounds the magnitude of potential harms of behavioral counseling interventions as no greater than small, based on the absence of reported harms in the literature and the noninvasive nature of the interventions (Table 2).
CESSATION
The USPSTF concludes that there is insufficient evidence to determine the balance of benefits and harms of primary care interventions for tobacco cessation among school-aged children and adolescents who already smoke because of a lack of adequately powered studies on behavioral counseling interventions and a lack of studies on medications.
The USPSTF found inadequate evidence on the benefit of behavioral counseling interventions for tobacco cessation in school-aged children and adolescents because many studies had small sample sizes and may not have been adequately powered to detect a benefit, making it unclear whether the observed lack of effect of interventions was the result of intervention failure or lack of statistical power. Although the USPSTF found no evidence on the harms of behavioral counseling interventions, it bounds the magnitude of potential harms of behavioral counseling interventions as no greater than small, based on the absence of reported harms in the literature and the noninvasive nature of the interventions.
The USPSTF found inadequate evidence on the benefits and harms of medications for tobacco cessation in children and adolescents, primarily because of an inadequate number of studies that have evaluated tobacco cessation medications in this population. Potential harms depend on the specific medication (Table 2).
Practice Considerations
PATIENT POPULATION UNDER CONSIDERATION
This recommendation applies to school-aged children and adolescents younger than 18 years. The USPSTF has issued a separate recommendation statement on interventions for tobacco use cessation in adults 18 years and older, including pregnant persons.16
DEFINITIONS
“Tobacco use” refers to use of any tobacco product. As defined by the U.S. Food and Drug Administration (FDA), tobacco products include any product made or derived from tobacco intended for human consumption (except products that meet the definition of drugs), including, but not limited to, cigarettes, cigars (including cigarillos and little cigars), dissolvable tobacco, hookah tobacco, nicotine gels, pipe tobacco, roll-your-own tobacco, smokeless tobacco products (including dip, snuff, snus, and chewing tobacco), vapes, e-cigarettes, hookah pens, and other electronic nicotine delivery systems. “Smoking” generally refers to the inhaling and exhaling of smoke produced by combustible tobacco products such as cigarettes, cigars, and pipes. “Vaping” refers to the inhaling and exhaling of aerosols produced by e-cigarettes.17 Vape products usually contain nicotine, which is the addictive ingredient in tobacco. Substances other than tobacco can also be used to smoke or vape.
ASSESSMENT OF RISK
All youth are considered at risk of initiating tobacco use. Interventions to prevent the initiation of tobacco use should be provided to all youth who have not started using tobacco products yet, regardless of the presence or absence of other risk factors. The following risk factors may increase the risk of tobacco use in youth: being male, white race, not college-bound, from a rural area, having parents with lower levels of education, parental smoking, having childhood friends who smoke, being an older adolescent, experiencing highly stressful events, and perceiving tobacco use as low risk.18,19
INTERVENTIONS TO PREVENT TOBACCO USE AND IMPLEMENTATION CONSIDERATIONS
Various behavioral counseling intervention types are effective in preventing tobacco initiation in children, including face-to-face counseling, telephone counseling, and computer-based and print-based interventions.14
Individual interventions target specific audiences (the child/adolescent, the parent, or both) and a variety of age ranges. For example, in the reviewed studies,14 interventions for children aged 7 to 10 years tended to be print-based materials, whereas face-to-face counseling and telephone- and computer-based interventions typically targeted children older than 10 years. Interventions targeting parents tended to be print- or telephone-based. The number of contacts made with intervention recipients also varied, ranging from 1 to 8 contacts.14 The intensity of the interventions varied, with the content of the print materials ranging from stickers to informational newsletters or an activity book (for children) or activity guide (for parents). For telephone-based interventions, telephone counseling was usually provided in conjunction with another modality such as print materials or face-to-face counseling. Based on the evidence reviewed,14 no specific component of behavioral counseling interventions (such as intervention modality, target audience, duration of intervention, or intervention setting) appeared to make an intervention more or less effective. Thus, clinicians have a broad range of effective behavioral counseling interventions from which to choose. For additional information about behavioral counseling interventions to prevent tobacco use, see Table 3,14,20–34 the Additional Tools and Resources section, and Table 4.
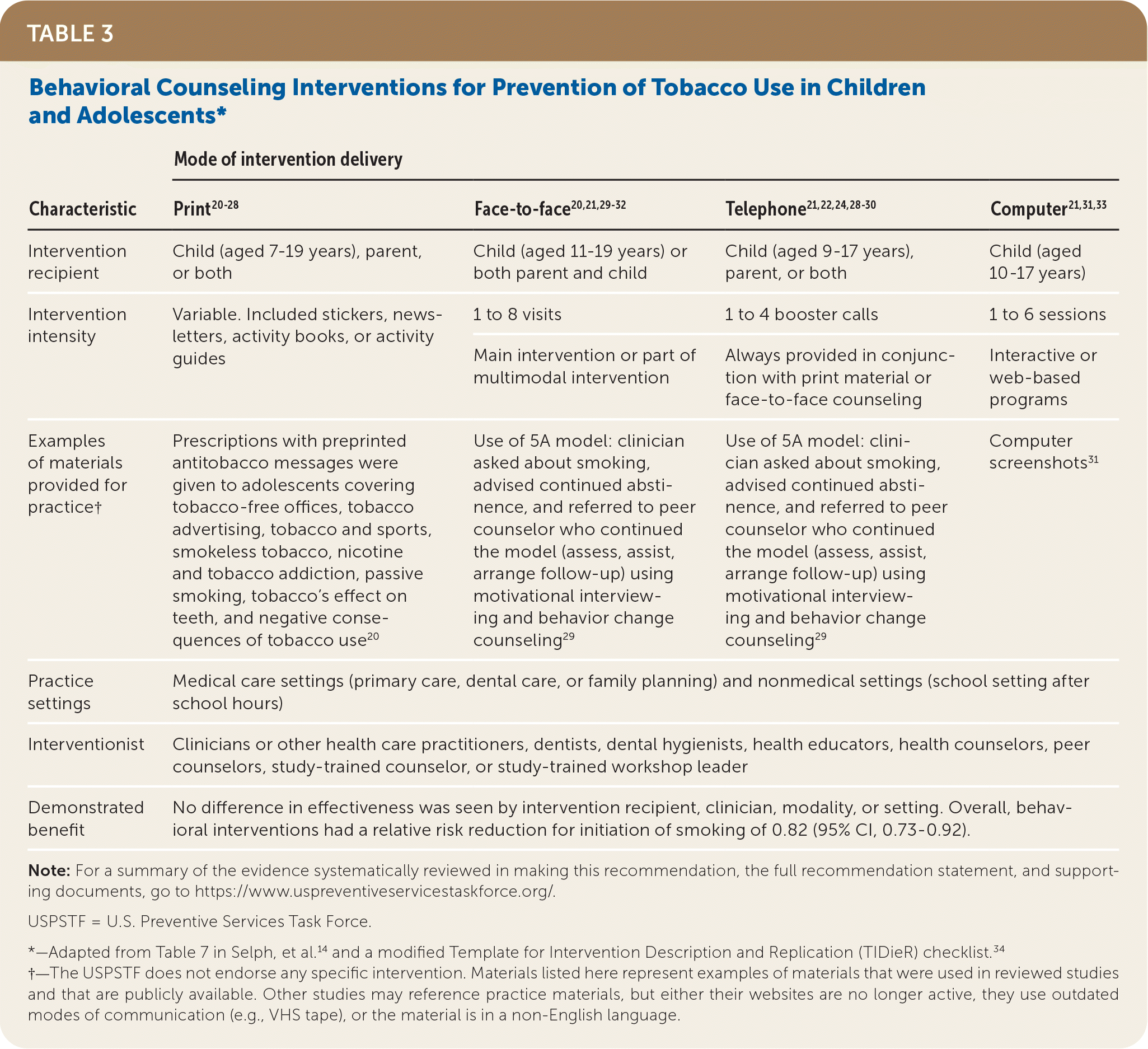
| Characteristic | Mode of intervention delivery | |||
|---|---|---|---|---|
| Print20–28 | Face-to-face20,21,29–32 | Telephone21,22,24,28–30 | Computer21,31,33 | |
| Intervention recipient | Child (aged 7–19 years), parent, or both | Child (aged 11–19 years) or both parent and child | Child (aged 9–17 years), parent, or both | Child (aged 10–17 years) |
| Intervention intensity | Variable. Included stickers, newsletters, activity books, or activity guides | 1 to 8 visits | 1 to 4 booster calls | 1 to 6 sessions |
| Main intervention or part of multimodal intervention | Always provided in conjunction with print material or face-to-face counseling | Interactive or web-based programs | ||
| Examples of materials provided for practice† | Prescriptions with preprinted antitobacco messages were given to adolescents covering tobacco-free offices, tobacco advertising, tobacco and sports, smokeless tobacco, nicotine and tobacco addiction, passive smoking, tobacco's effect on teeth, and negative consequences of tobacco use 20 | Use of 5A model: clinician asked about smoking, advised continued abstinence, and referred to peer counselor who continued the model (assess, assist, arrange follow-up) using motivational interviewing and behavior change counseling 29 | Use of 5A model: clinician asked about smoking, advised continued abstinence, and referred to peer counselor who continued the model (assess, assist, arrange follow-up) using motivational interviewing and behavior change counseling 29 | Computer screenshots31 |
| Practice settings | Medical care settings (primary care, dental care, or family planning) and nonmedical settings (school setting after school hours) | |||
| Interventionist | Clinicians or other health care practitioners, dentists, dental hygienists, health educators, health counselors, peer counselors, study-trained counselor, or study-trained workshop leader | |||
| Demonstrated benefit | No difference in effectiveness was seen by intervention recipient, clinician, modality, or setting. Overall, behavioral interventions had a relative risk reduction for initiation of smoking of 0.82 (95% CI, 0.73–0.92). | |||
Most of the evidence on behavioral counseling interventions to prevent tobacco use focused on prevention of cigarette smoking.14 Given the similar contextual and cultural issues currently surrounding the use of e-cigarettes in youth and the inclusion of e-cigarettes as a tobacco product by the FDA, the USPSTF concludes that the evidence on interventions to prevent cigarette smoking could be applied to prevention of e-cigarette use as well. The USPSTF also concludes that the evidence could be applied to prevention of cigar use, which includes cigarillos and little cigars.
ADDITIONAL TOOLS AND RESOURCES
Primary care clinicians may find the resources listed in the Table 4 useful in talking with children and adolescents about the harms of tobacco use.
OTHER RELATED USPSTF RECOMMENDATIONS
SUGGESTIONS FOR PRACTICE REGARDING THE I STATEMENT ON CESSATION
Potential Preventable Burden. Nearly 90% of adult daily smokers smoked their first cigarette by age 18 years.1 In 2019, an estimated 1.43 million high school and middle school students reported current use of cigars, 1.15 million high school and middle school students reported current use of conventional cigarettes, and 5.38 million high school and middle school students reported current use of e-cigarettes.7 Of high school and middle school students who used any tobacco product in the past 12 months, 57.5% reported that they had at least 1 quit attempt7; however, most quit attempts fail, and about 80% will go on to smoke into adulthood.36 Immediate adverse health effects in child and adolescent cigarette smokers include increased negative respiratory effects such as impaired lung growth, early onset of lung function decline, respiratory and asthma-related symptoms (e.g., coughing and wheezing), and early abdominal aortic atherosclerosis.1,36 Concerns regarding use of e-cigarettes in adolescence include nicotine dependence and toxicity, harm to the developing brain, its use as a bridge to conventional cigarette smoking, and inhalation of carcinogens.5
Although the evidence on behavioral counseling interventions to prevent tobacco use in children and adolescents is robust, fewer studies with smaller sample sizes are available that evaluate the effect of behavioral counseling interventions or pharmacotherapy on tobacco cessation.14 The pooled effect of the trials that evaluated behavioral counseling interventions for tobacco cessation in primary care settings did not find a significant reduction in smokers after the intervention.14 However, the study interventions were heterogeneous, and most of the studies were small, making it difficult to determine whether interventions were unsuccessful at helping children and adolescents to stop using tobacco or whether they were underpowered to detect a difference in tobacco cessation.
No medications are currently approved by the FDA for tobacco cessation in children and adolescents. The label for varenicline now states that it is not indicated in children and adolescents 16 years and younger because its efficacy in this population has not been demonstrated.37 Few trials have been published on medication use for tobacco cessation in children and adolescents (1 trial on nicotine replacement therapy [NRT] and 2 trials on bupropion sustained-release). Trials were relatively small, and all included behavioral counseling in addition to pharmacotherapy. None found a significant difference in quit rates at the end of treatment.14 One additional published trial on varenicline for cessation was identified; however, it was not considered in the USPSTF evidence review because the trial included young adults and the mean age of participants was older than the age in the inclusion criteria of the evidence review.
Given the insufficient evidence to identify effective interventions to help youth quit using tobacco, the USPSTF is calling for more research in this area.
Potential Harms. The USPSTF found no evidence on harms from behavioral counseling interventions for tobacco cessation14; however, these harms are likely small to none based on the absence of reported harms in the evidence, the noninvasive nature of the interventions, and the low likelihood of serious harms.
The USPSTF found the evidence on harms from medications for tobacco cessation in children and adolescents to be inadequate. None of the published trials reported any serious harms; however, study sizes were relatively small.14 The single trial of NRT found a greater number of headaches, cough, abnormal dreams, muscle pain, and patch-related adverse events with NRT.38 Bupropion carries a boxed warning for increased risk of suicidality in children, adolescents, and young adults, with other concerns for increased risk of seizure, hypertension, mania, visual problems, and unusual thoughts and behaviors.39 Varenicline is not indicated in children 16 years and younger; therefore, no warnings specific to this age group are included in its label.37 For older populations, labeling includes warnings and precautions for neuropsychiatric adverse events, including suicidality, seizures, interaction with alcohol, cardiovascular events, sleepwalking, angioedema, serious skin reactions, and nausea.37
This recommendation statement was first published in JAMA. 2020;323(16):1590–1598.
The “Update of Previous USPSTF Recommendation,” “Supporting Evidence,” “Research Needs and Gaps,” and “Recommendations of Others” sections of this recommendation statement are available at https://www.uspreventiveservicestaskforce.org/uspstf/recommendation/tobacco-and-nicotine-use-prevention-in-children-and-adolescents-primary-care-interventions.
The USPSTF recommendations are independent of the U.S. government. They do not represent the views of the Agency for Healthcare Research and Quality, the U.S. Department of Health and Human Services, or the U.S. Public Health Service.
