
Am Fam Physician. 2002;65(6):1123-1133
The treatment of osteosarcoma requires a multidisciplinary approach involving the family physician, orthopedic oncologist, medical oncologist, radiologist and pathologist. Osteosarcoma is a mesenchymally derived, high-grade bone sarcoma. It is the third most common malignancy in children and adolescents. The most frequent sites of origin are the distal femur, proximal tibia and proximal humerus. Patients typically present with pain, swelling, localized enlargement of the extremity and, occasionally, pathologic fracture. Most patients present with localized disease. Radiographs commonly demonstrate a mixed sclerotic and lytic lesion arising in the metaphyseal region of the involved bone. Computed tomography and bone scanning are recommended to detect pulmonary and bone metastases, respectively. Before 1970, osteosarcomas were treated with amputation. Survival was poor: 80 percent of patients died from metastatic disease. With the development of induction and adjuvant chemotherapy protocols, advances in surgical techniques and improvements in radiologic staging studies, 90 to 95 percent of patients with osteosarcoma can now be treated with limb-sparing resection and reconstruction. Long-term survival and cure rates have increased to between 60 and 80 percent in patients with localized disease.
Bone sarcomas are rare mesenchymally derived high-grade tumors. Approximately 2,500 cases per year are diagnosed in the United States.1 Osteogenic sarcoma is the most common bone sarcoma and the third most common malignancy in children and adolescents. The most frequent sites of origin are the metaphyseal regions of the distal femur, proximal tibia and proximal humerus, although the tumor can develop in any bone.2,3
Family physicians and pediatricians are often the first clinicians to encounter patients with osteosarcoma. Consequently, these physicians need to be familiar with the clinical presentation and overall management of this malignancy.
Most osteosarcomas are classified as conventional, high-grade tumors.2–4 Before 1970, amputation was the sole treatment for a high-grade osteosarcoma, and 80 percent of patients died of metastatic disease, most commonly to the lungs.2 Over the past three decades, effective induction (neoadjuvant/preoperative) and adjuvant (postoperative) chemotherapy protocols have improved the ability to perform safe limb-sparing resections, and disease-free and overall survival rates have risen. Today, 90 to 95 percent of patients with osteosarcoma can be treated with limb-sparing surgery, and 60 to 80 percent of patients with localized disease are long-term survivors.5–7
Biologic Behavior of Osteosarcoma
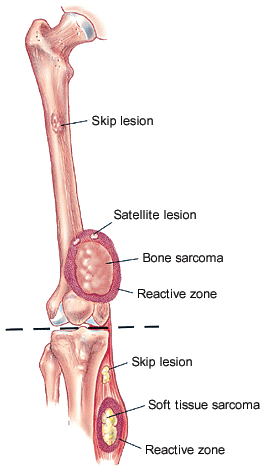
An osteosarcoma grows in a radial manner, forming a ball-like mass. When it penetrates the bony cortex, it compresses the surrounding muscles into a pseudocapsular layer referred to as the “reactive zone.” Tumor nodules representing microextensions of the primary mass invade the reactive zone. These nodules are termed “satellites.” The entire tumor mass, including the reactive zone (satellites), must be resected to ensure removal of all gross tumor. Thus, the surgical margin must be wide.
The tumor may metastasize regionally (within the same extremity) or systemically (to other organs, such as the lung). With metastasis, the prognosis worsens dramatically. Tumor nodules growing outside the reactive rim but within the same bone or across a neighboring joint are termed “skip lesions” and represent regional intraosseous or transarticular metastases, respectively (Figure 1).8 Systemic metastases have a predilection for the lungs. The bones are the second most common site of metastasis and usually become involved only after pulmonary metastases have occurred. Distant bone metastases represent the latest stage of disease and are associated with the poorest prognosis.9–11
Clinical Presentation
Most conventional osteosarcomas develop in patients 10 to 20 years of age (Table 1). Approximately 80 percent of these tumors are nonmetastatic at the time of presentation. A high index of suspicion for osteosarcoma enables early diagnosis, which can have a positive effect on survival.
| History |
| Patient age: 5 to 30 years (most commonly, 10 to 20 years) |
| Dull, aching pain (occasionally, referred pain, especially in children) |
| Night pain, “growing pains” |
| History of minor trauma, injury, sprain or muscle strain |
| Fever, night sweats, weight loss* |
| Physical examination |
| Local tenderness |
| Swelling, mass, deformity |
| Limp, muscle atrophy, decreased joint motion |
| Pathologic fracture |
| Lymphadenopathy* |
Patients typically present with dull, aching pain of several months' duration that may suddenly become more severe. The increase in pain severity may correlate with tumor penetration of cortical bone and irritation of the periosteum, or with pathologic fracture. Night pain is common and may awaken the patient from sleep. Hence, chronic indolent night pain should not be dismissed as “growing pains,” especially when it is unilateral. Patients frequently have a history of a minor injury, sprain or muscle pull incurred while participating in a sport.
The physical examination may reveal localized tenderness, restricted range of motion of the adjacent joint, a limp or muscle atrophy, and may confirm the presence of a mass, swelling or deformity. Children frequently have referred pain; therefore, it is essential to perform a comprehensive examination of the joint above and below the area of complaint, as well as spinal and reflex examinations.
Regional lymph node metastases are rare in patients with osteosarcoma. Enlarged, tender regional lymph nodes are more suggestive of osteomyelitis, which should be considered in the differential diagnosis.
Radiologic Studies
Radiologic studies that are recommended for evaluation of the primary tumor and detection of metastatic disease are discussed in the following sections and summarized in Table 2.
| Imaging modality | Purpose | Study findings at presentation | Changes consistent with good response to induction chemotherapy |
|---|---|---|---|
| Plain-film radiography | Differential diagnosis Estimate effects of chemotherapy Detect pulmonary metastasis | Most commonly, mixed Chest nodules or “cannonball”lesions | Increased ossification Periosteal thickening and new bone formation Increased sclerosis of tumor border Decreased size of soft tissue mass |
| MRI | Determine extent of tumor Detect soft tissue mass or skip lesion Determine relationship of tumor to neurovascular bundle | Length of bone affected Size of soft tissue mass No findings specifically characteristic of osteosarcoma | Not accurate; however, the MRI study may show a thick, dark rim around the tumor, which is consistent with periosteal new bone formation. |
| CT of affected extremity | Determine extent of tumor, especially in the presence of excessive tumoral edema Visualize vessels (using contrast medium) | Mixed sclerotic and lytic lesion | Increased ossification Rim of calcification surrounding tumor Reduction in size of soft tissue mass |
| CT of chest | Detect pulmonary metastasis | Small nodules or large cannonball lesions (late stage) | Increased ossification Decrease in size or disappearance of tumor nodules |
| Bone scintigraphy | Determine sites of bone metastasis Detect intraosseous extension | Increased bony uptake | Not accurate; new bone formation may yield increased uptake. Flow study shows decreased vascularity. |
| Thallium scintigraphy | Monitor effects of chemotherapy Detect local recurrence of tumor | Increased uptake in tumor | Complete disappearance in uptake |
| Angiography | Determine vascularity of tumor Detect vascular displacement and determine relationship of vessels to the tumor Identify vascular anomalies Estimate effects of chemotherapy | Neovascularization Tumor blush Vascular anomalies | Complete disappearance of neovascularity and tumor blush |
PLAIN-FILM RADIOGRAPHS
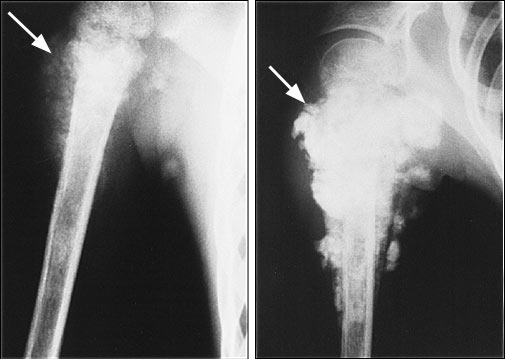
Most conventional osteosarcomas originate in the metaphyseal region of bone and present radiographically as mixed sclerotic and lytic lesions (Figure 2), although primarily sclerotic or lytic lesions also occur (Figure 3). Sclerosis is the result of tumor osteoid production and typically appears as small, irregular, cloud-like densities. Most osteosarcomas present with destruction of the bony cortex and the formation of a soft tissue mass. The soft tissue component may demonstrate ossification and thus may be detectable on radiographs. Pathologic fractures can also be identified.
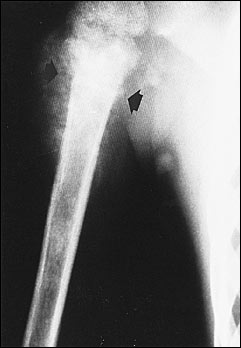
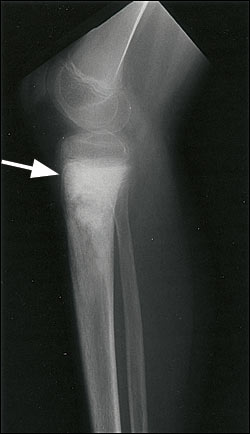
Radiographs are used to differentiate among the possible causes of a bone mass (Table 3). They are also used to detect metastatic pulmonary disease, although computed tomographic (CT) scanning of the chest is more sensitive for this purpose.
| Osteosarcoma |
| Ewing's sarcoma |
| Osteomyelitis |
| Osteoblastoma |
| Giant cell tumor |
| Aneurysmal bone cyst |
| Fibrous dysplasia |
MAGNETIC RESONANCE IMAGING
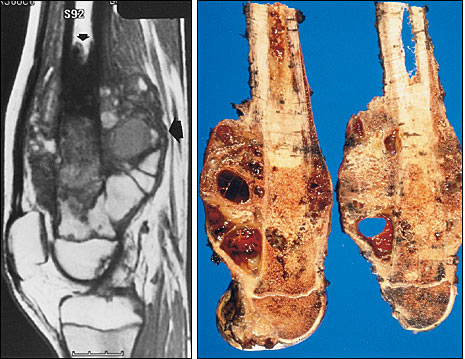
Based on the MRI studies, the relationship of the tumor to the neurovascular bundle can be determined, and resectability can be estimated. Neurovascular encasement is rare; when present, it generally necessitates amputation or wide resection with vascular reconstruction.
Obtaining an MRI study at the time of surgical resection permits accurate planning of the osteotomy site through the involved bone, 2 to 3 cm away from the tumor, for the purpose of achieving a wide surgical margin. Skip lesions that occur within the same bone or across the adjacent joint (transarticular skip metastases) are readily identified on MRI scans. When skip lesions are present, more extensive resection is required.
CT SCANNING
CT scanning of the affected extremity is useful in demonstrating the extraosseous and intraosseous extent of the tumor. When extensive necrosis and surrounding edema are present, CT scanning may be superior to MRI for accurately determining the extent of soft tissue involvement and the proximity of the tumor to neurovascular structures. When contrast medium is used, adjacent vascular structures can be identified.
All patients with osteosarcoma should undergo CT scanning to detect metastatic pulmonary disease. After surgery has been performed in patients with nonmetastatic tumors, CT studies should be repeated every three to six months for two years. (Most distant metastases occur within two years after the termination of treatment.) Patients who present with metastatic pulmonary disease have a much poorer prognosis. However, cure can be achieved in a small number of patients who respond well to chemotherapy and undergo pulmonary metastatectomy.10,11,14
BONE SCINTIGRAPHY
Triple-phase, whole-body bone scintigraphy can help determine the sites of metastatic disease, polyostotic involvement and the intraosseous extent of the tumor. It may also detect skip lesions, although MRI is more accurate for this purpose. Nuclear medicine scintigraphy can image the entire axial and appendicular skeleton to detect distant sites of osseous metastases.
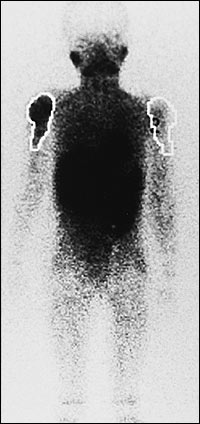
THALLIUM SCINTIGRAPHY
Thallium Tl 201 is a potassium analog that is actively transported via the sodium-potassium adenosine triphosphatase pump. This radioisotope, which accumulates in both benign and malignant tumors, reflects tumor metabolic activity. Thallium scintigraphy is useful for monitoring the response of a tumor to induction chemotherapy and for detecting local recurrence, especially when MRI is not helpful15 (Figure 5).
ANGIOGRAPHY
Angiography can assist in surgical planning and in estimating the response of the tumor to preoperative chemotherapy.16 High-grade osteosarcomas promote extensive neovascularization and thus enhance with contrast medium. This enhancement, referred to as “tumor blush,” usually reflects viable tumor. The complete disappearance of tumor vascularity after preoperative chemotherapy correlates with a good treatment response.
Biplanar angiography accurately determines the relationship of vessels to the tumor, along with the vascular displacement that occurs with a large soft tissue component. Vascular anomalies can also be detected, which is especially important for resection of proximal tibial tumors, in which the anterior tibial artery is routinely ligated. Arteriography is essential to ensure the presence and patency of the posterior tibial artery before ligation of the anterior tibial artery.
Angiography is an invasive study. Depending on the surgical complexity of the case and the patient's response to induction chemotherapy as determined by other less invasive studies, some surgeons may decide to forego angiography.
Biopsy
Biopsy is the key step in the diagnosis of an osteosarcoma. Improperly performed biopsies are a frequent cause of misdiagnosis, amputation and local recurrence, and they may have a negative effect on survival. All biopsy samples should be obtained by the orthopedic oncologist who will perform the definitive procedure or by a physician directly under the oncologist's supervision.
Staging studies are helpful in planning the surgical approach to the tumor and specifying the region of the tumor that will most likely yield representative pathologic material. In many instances, a percutaneous needle biopsy may be recommended, because it is minimally invasive, does not require wound healing and is associated with a lower risk of infection, contamination and postbiopsy fracture.
Pathology and Staging
Osteosarcomas can be divided into high-grade and low-grade variants, depending on cellularity, pleomorphism, anaplasia and number of mitoses. Tumor osteoid production is characteristic of each subtype. Microscopically, conventional osteosarcomas are composed of malignant-appearing spindle cells that produce osteoid.
Chemotherapy
Chemotherapy is vital in the treatment of osteosarcoma. Advances in chemotherapy over the past 30 years have been responsible for improved limb salvage and higher survival rates.5–7,18,19 Chemotherapy has also been shown to reduce the number of pulmonary metastases or to delay their appearance, possibly facilitating surgical removal.20
Standard regimens now include preoperative (induction) and postoperative (adjuvant) chemotherapy. Preoperative chemotherapy induces tumor necrosis in the primary tumor and provides early treatment of micrometastatic disease. It helps to facilitate surgical resection with wide margins and therefore has been one of the main factors contributing to improved limb salvage rates.
Drugs that have been shown to be most effective against osteosarcoma include doxorubicin (Adriamycin), cisplatin (Platinol), ifosfamide (Ifex) with mesna (Mesnex) and high-dose methotrexate (Rheumatrex) with leucovorin calcium rescue. The mechanisms of action and side effects of these chemotherapeutic agents are summarized in Table 5.
| Agent | Mechanism of action | Side effects |
|---|---|---|
| Doxorubicin (Adriamycin) | Doxorubicin intercalates at points of local uncoiling of the DNA double helix; it also inhibits the synthesis of DNA and RNA. | Cardiomyopathy, transient electrocardiographic abnormalities, emesis, alopecia, mucositis, myelosuppression |
| Cisplatin (Platinol) | Cisplatin inhibits the synthesis of DNA through the formation of DNA cross-links; it binds directly to tumor DNA and denatures the DNA double helix. | Acute renal failure, chronic renal failure, peripheral neuropathy, ototoxicity, emesis, myelosuppression, alopecia, hypomagnesemia |
| Ifosfamide (Ifex), with mesna (Mesnex)* | Ifosfamide causes cross-linking of DNA strands, inhibiting the synthesis of DNA and protein. | Hemorrhagic cystitis, renal failure, myelosuppression, alopecia, emesis, encephalopathy |
| High-dose methotrexate (Rheumatrex), with leucovorin calcium rescue† | Methotrexate is a folate antimetabolite; it inhibits the synthesis of purine and thymidylic acid by binding dihydrofolate reductase. | Renal failure, mucositis, mild myelosuppression; rarely, central nervous system effects |
Most standard protocols use doxorubicin and cisplatin, with or without high-dose methotrexate, for both induction and adjuvant chemotherapy. Recent trials have incorporated ifosfamide into their protocols. However, the benefit of ifosfamide over conventional regimens in improving patient survival has not yet been confirmed.
Modern multiagent, dose-intensive chemotherapy regimens have resulted in long-term disease-free survival rates of approximately 60 to 80 percent in patients who present with localized (nonmetastatic) disease.6,7 The best long-term survival statistics have been reported in patients who achieved greater than 90 percent histologic tumor necrosis in the resected specimen.5,21–23 Consequently, the amount of tumor necrosis achieved with induction chemotherapy is routinely estimated for all specimens following resection.
Radiologic Estimation of Tumor Response to Induction Chemotherapy
Preoperative radiologic assessment of the response of the tumor to induction chemotherapy permits an estimation of prognosis and facilitates surgical planning. Patients who demonstrate a poor response require a wider margin of resection. Presently, radiography, CT scanning, thallium scintigraphy and angiography are the most useful tests for estimating the response to induction chemotherapy, and their findings should be interpreted collectively (Figure 6).
Surgery
“Limb salvage” refers to successful resection of a tumor and reconstruction of a viable, functional extremity. In the setting of induction chemotherapy, limb-sparing resection and reconstruction, rather than amputation, can be safely performed in 90 to 95 percent of patients.6 Studies indicate that compared with amputation, limb-sparing surgery using a wide margin does not appear to compromise survival.24,25
It should be emphasized, however, that the primary objective of overall treatment is to achieve long-term disease-free survival (cure). Preserving limb function is a secondary objective. If an adequate limb-sparing resection cannot be performed, amputation should be considered. However, with modern chemotherapy regimens, limb removal is seldom necessary.
After tumor resection, the large bony and soft tissue deficit must be reconstructed. Many orthopedic surgeons prefer metallic endoprostheses for reconstruction. These prostheses provide immediate stable fixation and allow early ambulation and weight bearing. They provide joint stability, with good to excellent function in most patients.26–29 Metallic endoprostheses are associated with only minimal early postoperative complications.
Postoperative Follow-up
After chemotherapy has been completed, the patient should be followed closely by the orthopedic oncologist and medical oncologist. Occasionally, this may not be feasible, and follow-up then becomes the responsibility of the family physician.
The patient should be monitored for local and systemic recurrence, as well as complications related to the reconstruction. The most common complications associated with prosthetic reconstruction are loosening, infection and mechanical failure.
CT scanning of the chest, plain-film radiography of the reconstructed extremity and serial physical examinations are recommended every three months for the first two years after treatment, at least every six months from the second through the fifth years, and subsequently on a yearly basis. Annual bone scintigraphy is recommended for the first two years after treatment.
During physical examinations, the extremity should be palpated carefully for masses. Patient complaints of extremity pain, joint instability, joint effusion, prosthetic locking and warmth or redness of the extremity may represent prosthetic failure, loosening or infection. Radiographic findings of progressive radiolucent lines surrounding the prosthetic stem or areas of osteolysis also suggest prosthetic loosening or infection. If any concerns arise, the patient should be promptly referred to the orthopedic oncologist.