Am Fam Physician. 2019;99(10):643-648
Author disclosure: No relevant financial affiliations.
Glycopyrrolate nebulizer solution (Lonhala Magnair) is labeled for long-term maintenance therapy of chronic obstructive pulmonary disease (COPD), including chronic bronchitis and emphysema. It is available with a portable nebulizer for self-administration by patients.
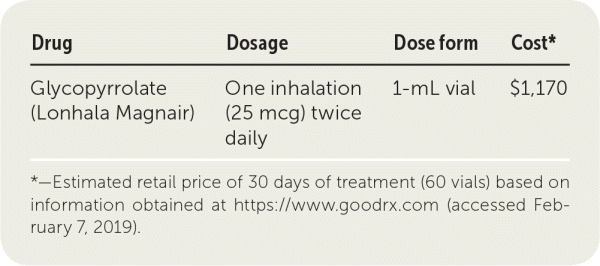
| Drug | Dosage | Dose form | Cost* |
|---|---|---|---|
| Glycopyrrolate (Lonhala Magnair) | One inhalation (25 mcg) twice daily | 1-mL vial | $1,170 |
Safety
Glycopyrrolate is an anticholinergic, which could theoretically induce narrow-angle glaucoma and worsen symptoms of urinary retention. Glycopyrrolate should not be used with other medications that have anticholinergic activity.1 It should not be initiated in patients during an acute exacerbation of COPD. All patients started on glycopyrrolate should have a short-acting beta agonist for treatment of acute symptoms and paradoxical bronchospasm. Glycopyrrolate should be avoided in patients with unstable cardiac disease because studies have not evaluated safety. Glycopyrrolate is excreted in breast milk and should not be used in pregnant and lactating women unless the expected benefit outweighs the potential risk. It is not approved for use in children.
Tolerability
Glycopyrrolate is well tolerated. In clinical trials, the pooled dropout rate due to adverse effects was 5%, compared with 9% for patients treated with placebo. Two 12-week randomized, placebo-controlled trials involved a total of 431 patients with moderate to severe COPD (average forced expiratory volume in one second [FEVI] of 52% of predicted). More than 2% of participants experienced adverse effects, including dyspnea (4.9% vs. 3% with placebo) and urinary tract infection (9% vs. 6% with placebo).1 No adjustment in dosage is necessary in older patients or in patients with hepatic impairment or mild to moderate renal disease.
Effectiveness
When added to treatment with a long-acting beta agonist, with or without an inhaled corticosteroid and ipratropium (Atrovent), glycopyrrolate will increase FEV1 by 81 to 96 mL, which is similar to the increase achieved with tiotropium (Spiriva).1,2 The effectiveness of glycopyrrolate was confirmed in a larger 48-week open-label study.3 Quality of life is significantly improved in one additional patient for every nine patients treated with glycopyrrolate vs. placebo, but glycopyrrolate has not been studied as the sole long-acting therapy for COPD.
Glycopyrrolate has not been evaluated in patients with mild COPD, and studies have not determined its effect on patient-oriented outcomes (e.g., risk of exacerbation, hospitalization).
Price
A one-month supply of glycopyrrolate (60 vials) costs approximately $1,170. This price includes the cost of the nebulizer for administration. In comparison, a one-month supply of tiotropium costs about $445.
Simplicity
The recommended dosage of glycopyrrolate is one 25-mcg inhalation twice daily. Patients should be instructed in the administration of glycopyrrolate as well as assembly and care of the nebulizer. Administration requires two to three minutes. No research has compared its ease of use with administration of a metered-dose inhaler.
Bottom Line
Glycopyrrolate is a safe alternative to long-acting muscarinic antagonists delivered by metered-dose inhaler for patients with moderate to severe COPD. However, it is significantly more expensive compared with other treatments. Its use should be limited to patients who cannot use a metered-dose inhaler.