
Am Fam Physician. 2021;104(2):209-210
Author disclosure: No relevant financial affiliations.
Key Points for Practice
• During a second gout flare-up in one year, low-dose allopurinol can be started with anti-inflammatory therapy without worsening the flare-up.
• Allopurinol is the preferred urate-lowering agent, but HLA testing should be offered to patients of Southeast Asian or African American descent before starting to identify patients at risk for an allergic reaction.
• Titrating urate-lowering therapy to reach a serum urate level of 6 mg per dL decreases flare-ups and increases treatment adherence.
• During acute flare-ups, low-dose colchicine, NSAIDs, and glucocorticoids delivered orally, intramuscularly, or intra-articularly are similarly effective.
From the AFP Editors
Although effective medications exist to prevent and treat acute flare-ups, gout remains the most common inflammatory arthritis in the United States. Urate-lowering therapy is underused despite previous recommendations from the American College of Rheumatology (ACR). The ACR published updated guidelines for gout management focused on improving prevention of flare-ups.
When to Consider Urate-Lowering Therapy
Urate-lowering therapy is recommended for patients with two or more gout flare-ups per year, tophaceous gout, or damage attributable to gout visible on radiography. Consider starting therapy for patients with a second flare-up even if not within one year. With a first gout flare-up, shared decision-making is appropriate in patients at high risk because of a serum urate level of 9 mg per dL (0.54 mmol per L) or higher, urolithiasis, or stage 3 or greater chronic kidney disease. Urate-lowering therapy has not been shown to be beneficial for patients with asymptomatic hyperuricemia.
The ACR recommends starting urate-lowering therapy during an acute flare-up instead of waiting until it resolves. Therapy started during a flare-up does not increase or prolong symptoms as long as anti-inflammatory treatments are provided. The ACR notes that patients are most motivated for treatment during an exacerbation. Anti-inflammatory medications such as colchicine or nonsteroidal anti-inflammatory drugs (NSAIDs) should be continued for three to six months after starting urate-lowering therapy.
Urate-Lowering Therapies
Allopurinol is the first-line urate-lowering therapy. Febuxostat (Uloric), the other xanthine oxidase inhibitor, is limited by increased cardiovascular and all-cause mortality seen in studies. Hypersensitivity reactions to allopurinol occur in patients with the HLA-B*5801 allele. Because this allele affects 7% of people of Southeast Asian descent and 4% of people of African American descent, HLA testing is recommended for these patients before starting allopurinol. Allopurinol desensitization can be performed after an allergic response. Urate-lowering therapies are summarized in Table 1.
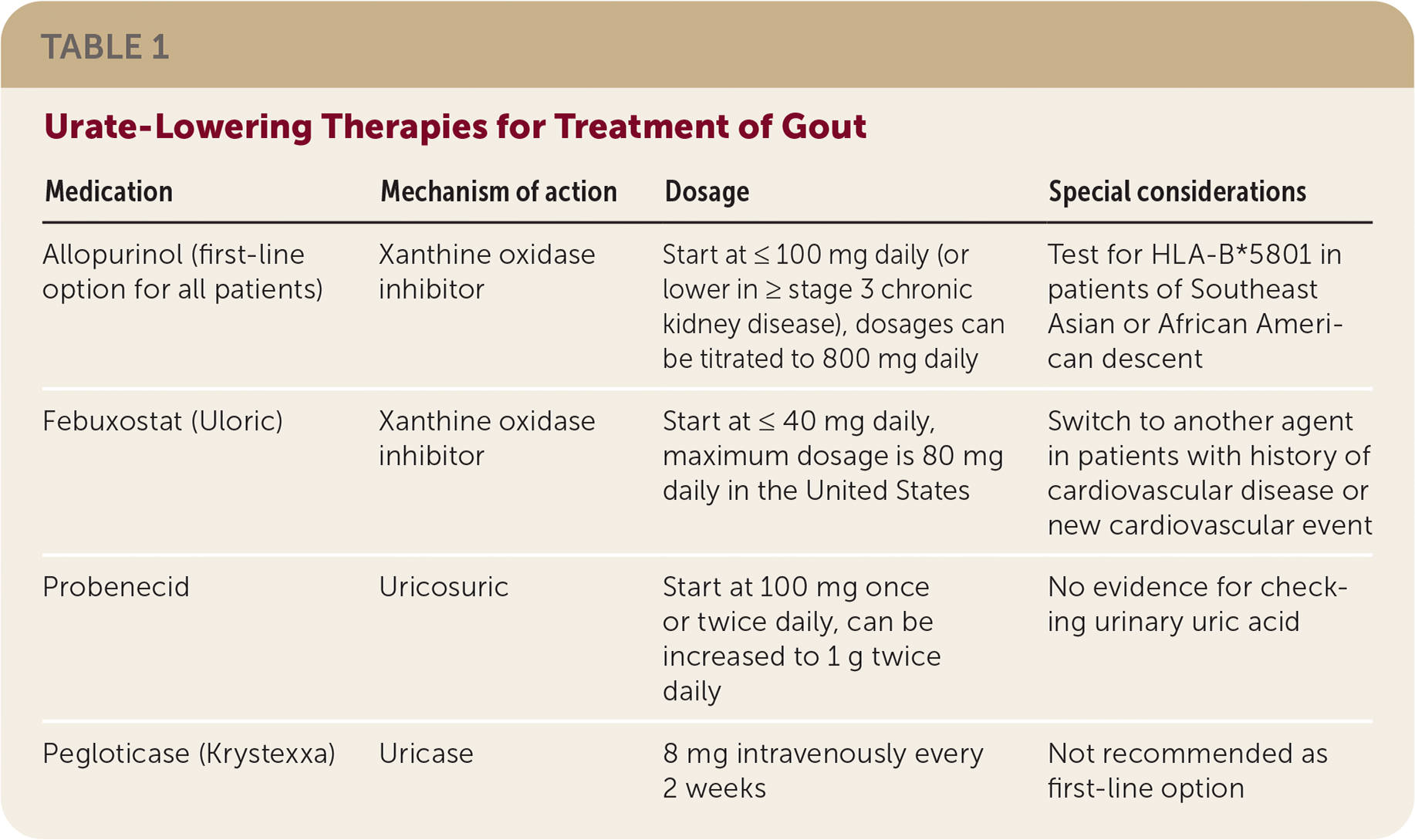
| Medication | Mechanism of action | Dosage | Special considerations |
|---|---|---|---|
| Allopurinol (first-line option for all patients) | Xanthine oxidase inhibitor | Start at ≤100 mg daily (or lower in ≥ stage 3 chronic kidney disease), dosages can be titrated to 800 mg daily | Test for HLA-B*5801 in patients of Southeast Asian or African American descent |
| Febuxostat (Uloric) | Xanthine oxidase inhibitor | Start at ≤40 mg daily, maximum dosage is 80 mg daily in the United States | Switch to another agent in patients with history of cardiovascular disease or new cardiovascular event |
| Probenecid | Uricosuric | Start at 100 mg once or twice daily, can be increased to 1 g twice daily | No evidence for checking urinary uric acid |
| Pegloticase (Krystexxa) | Uricase | 8 mg intravenously every 2 weeks | Not recommended as first-line option |
Consider starting allopurinol at 100 mg or less daily and febuxostat at 40 mg or less daily. Dosing should be increased every two to five weeks to reduce serum urate levels to 6 mg per dL (0.36 mmol per L) or less. Titrating medication slowly increases adherence while reducing flare-ups and tophi. Urate-lowering therapy may have to be continued for life. In a study of patients with low serum urate levels, 87% had flare-ups within five years of stopping urate-lowering therapy.
Probenecid is minimally effective, so consider switching to febuxostat instead of adding probenecid for poor control on the highest tolerated dose of allopurinol.
Pegloticase (Krystexxa) is an intravenous medication that breaks down urate. Because of high cost and adverse effects, pegloticase is a poor choice for initial therapy. It can be considered for patients who have tophi or more than one flare-up per year despite maximally tolerated therapy.
Acute Flare-up Management
Several medications are effective for acute flare-ups. The effectiveness of low-dose colchicine is similar to that of high-dose colchicine with fewer adverse effects. NSAIDs and glucocorticoids are as effective as colchicine. Glucocorticoids can be given intramuscularly, intra-articularly, or orally. Interleukin-1 inhibitors and adrenocorticotropic hormone have less evidence and higher cost. Limited evidence suggests topical ice is helpful as an adjuvant treatment.
Concurrent Medications and Lifestyle Modifications
Patients with hypertension and gout should be switched from hydrochlorothiazide to losartan (Cozaar) if possible. Low-dose aspirin should be continued if indicated, even though aspirin increases urate levels. Although fenofibrate (Tricor) reduces urate levels, changing cholesterol therapy is not recommended for gout.
There is limited evidence that restricting consumption of alcohol, purines, and high fructose corn syrup reduces urate levels, whereas vitamin C supplementation is not effective. Weight loss reduces gout flare-ups. The benefit from dietary changes is limited, and may be outweighed by the risk that patients may feel blamed.
Guideline source: American College of Rheumatology
Evidence rating system used? Yes
Systematic literature search described? Yes
Guideline developed by participants without relevant financial ties to industry? No
Recommendations based on patient-oriented outcomes? Yes
Published source: Arthritis Care Res (Hoboken). June 2020;72(6):744–760 [published corrections appear in Arthritis Care Res (Hoboken). 2020;72(8):1187, and Arthritis Care Res (Hoboken). 2021;73(3):458]
Editor’s Note: While this guideline recommends important changes to the standard treatment of gout, the recommendation that certain racial groups receive HLA-B*5801 testing before receiving allopurinol is problematic. A recent commentary in JAMA points out that race poorly discriminates risk of allergic reaction, since the prevalence of the HLA-B*5801 allele varies dramatically within geographic and ethnic groups.1 For example, HLA-B*5801 is common in Thailand, China, and South Korea, but rare in Japan. Variation across populations in Africa is similar, ranging from low rates comparable to Japan to higher rates comparable to Thailand. As Dr. Reddick notes in his editorial in this issue (https://www.aafp.org/afp/2021/0800/p122.html) “Although we may hear about biologic or genetic differences between races, there is more variation within races than there is between them.” In counseling our patients about the recommendations for HLA testing, it iss important to remember that categorization by race is an imprecise predictor of risk. Until we have more accurate tools to differentiate risk, shared decision making is imperative.-Michael J. Arnold, Contributing Editor.
Reference
1. Goodman CW, Brett AS. Race and pharmacogenomics—personalized medicine or misguided practice? JAMA. 2021;325(7):625-626.
