
Am Fam Physician. 1999;60(8):2269-2276
The first episode of nephrolithiasis provides an opportunity to advise patients about measures for preventing future stones. Low fluid intake and excessive intake of protein, salt and oxalate are important modifiable risk factors for kidney stones. Calcium restriction is not useful and may potentiate osteoporosis. Diseases such as hyperparathyroidism, sarcoidosis and renal tubular acidosis should be considered in patients with nephrolithiasis. A 24-hour urine collection with measurement of the important analytes is usually reserved for use in patients with recurrent stone formation. In these patients, the major urinary risk factors include hypercalciuria, hyperoxaluria, hypocitraturia and hyperuricosuria. Effective preventive and treatment measures include thiazide therapy to lower the urinary calcium level, citrate supplementation to increase the urinary citrate level and, sometimes, allopurinol therapy to lower uric acid excretion. Uric acid stones are most often treated with citrate supplementation. Data now support the cost-effectiveness of evaluation and treatment of patients with recurrent stones
After an episode of acute urolithiasis, patients are particularly motivated to learn about preventive strategies. Because kidney stones affect as many as 15 percent of men and 7 percent of women in the United States,1 family physicians have frequent opportunities to dispense preventive advice. Compared with urology practices, family practice settings are more conducive to and appropriate for the dissemination of requisite recommendations on the prevention of kidney stones.
| Evaluation of patient with first stone episode |
| History: medications, occupation, family history of stones or other kidney disease, inflammatory bowel Disease (e.g., Crohn's disease) |
| Diet: intake of protein, purines, sodium, fluids, oxalate and calcium |
| Laboratory tests: electrolyte, blood urea nitrogen, creatinine, calcium, phosphate and uric acid levels, urinalysis, urine culture if indicated, stone analysis if available (if not, consider qualitative cystine screening) |
| Radiology: plain radiographs, ultrasonography and/or intravenous pyelography (or helical computed tomography) to find more stones, radiolucent stones or anatomic abnormalities |
| Consider: renal tubular acidosis, hyperparathyroidism and sarcoidosis |
| Evaluation of patient with recurrent stone formation (and all children) |
| Twenty-four–hour urine collection: volume, pH, levels of calcium, phosphorus, sodium, uric acid, oxalate, citrate, creatinine, calcium oxalate (supersaturation), calcium phosphate and uric acid |
| Repeat as necessary: 24-hour urine collection and analysis to monitor response to dietary changes and effectiveness of treatment |
First Stone Episode
Approximately 80 percent of kidney stones contain calcium. The majority of these stones are composed of calcium oxalate, with a minority containing calcium phosphate or admixtures of oxalate and phosphate salts. About 10 percent of stones are composed of uric acid (sometimes associated with a history of gout) or mixed uric acid and calcium. Another 10 percent are struvite stones, which develop exclusively in patients with urinary tract infections caused by urease-producing organisms, most typically Proteus species. Cystine accounts for no more than 1 percent of all stones. Cystine stones arise only in patients with cystinuria, an autosomal recessive disorder.1
Stone analysis is inexpensive (about $25) and is worth performing for first stones, those formed during preventive treatment and those occurring in conjunction with infection. If no stone is available for analysis, qualitative screening for urinary cystine should be performed at least once in younger patients.
Risk factors for nephrolithiasis are summarized in Table 2. Identifying a familial incidence of stones is useful because it indicates an increased risk of recurrence. Environmental or occupational factors and bowel surgeries such as ileostomy may be predisposing factors because of low urine volumes. Residents of the “stone belt” in the southeastern part of the United States also appear to be at higher risk for stone formation.3 In the stone belt, two mechanisms have been implicated: the hot climate causes increased perspiration and reduced urine volume, and exposure to sunlight activates vitamin D, stimulating the absorption of dietary calcium.
| Male gender (men constitute about two thirds of stone formers) | |
| Increasing age (risk increases until the age of 65 years) | |
| Low urine volume | |
| Occupational or situational factors: inadequate access to bathroom facilities or drinking water (e.g., delivery persons, sales persons), athletic activity, heat and sun exposure | |
| Bowel disease | |
| Geographic factors: residence in the “stone belt” of southeastern United States, or Mediterranean or Middle Eastern countries | |
| Hereditary factors and disorders: polycystic kidney disease, renal tubular acidosis, hyperparathyroidism, cystinuria, hypocitraturia, hypercalciuria | |
| Other renal disorders: infection (struvite calculus), medullary sponge kidney | |
| Dietary factors: increased intake of protein, salt or oxalate, decreased intake of calcium | |
| Hypercalciuria: hypercalcemia (hyperparathyroidism, sarcoidosis), increased intestinal absorption of calcium, renal leakage of calcium or phosphate, release of calcium from bone | |
| Hyperuricosuria: increased risk for calcium or urate stones | |
| Hyperoxaluria: primary hyperoxaluria, dietary intake of oxalate, enteric hyperoxaluria | |
| Hypocitraturia: increased protein intake, idiopathic | |
| Acidosis: acetazolamine (Diamox), renal tubular acidosis, bowel disease, protein loading | |
Drugs associated with stone formation include triamterene (Dyrenium) and the sulfonamides, which have low solubility. Calcium and vitamin D supplements cause hypercalciuria, and carbonic anhydrase inhibitors, which are used to treat glaucoma, increase the urinary pH and precipitate calcium phosphate. Indinavir (Crixivan), a protease inhibitor, can also crystallize and form stones in the urinary tract.4
Animal protein is a major dietary constituent responsible for the relatively high prevalence of stones in populations of developed countries. Several mechanisms have been identified. Protein ingestion increases renal acid excretion. This, in turn, increases renal reabsorption of potential base, such as citrate, which is an endogenous inhibitor of calcium stone formation. Acid loads may be buffered in part by bone, which releases calcium to be excreted by the kidney. Finally, acid loading directly inhibits renal calcium reabsorption.5
Animal protein is also the major dietary source of purines, the precursors of uric acid. Excessive intake of animal protein is therefore associated with hyperuricosuria, a condition present in some uric acid stone formers. More importantly, uric acid solubility is largely determined by the urinary pH. As the pH falls below 5.5 to 6.0, the solubility of uric acid decreases, and uric acid precipitates, even if hyperuricosuria is not present.7 One last important link between dietary protein and stones is the decrease in calcium oxalate solubility caused by uric acid. As a result, hyperuricosuria is also associated with calcium stone formation.
Another dietary risk factor for stones is sodium ingestion, although no controlled studies have shown that sodium restriction prevents stone formation. Increases in urinary sodium excretion cause increased urinary calcium excretion through renal mechanisms and increased calcium mobilization from bone.6 Higher sodium excretion rates also increase uric acid excretion and decrease urinary citrate excretion. Therefore, patients should be warned about added table salt and the salt content of processed meats, cheese, canned foods, soy sauce, baked goods and restaurant food.
Endogenous metabolism contributes a larger proportion of urinary oxalate than does dietary intake. Nonetheless, patients should be asked about their intake of foods with a high oxalate content, such as nuts, chocolate and dark-green leafy vegetables. Rhubarb, beets and okra are especially high in oxalate. Tomato sauce and jams are concentrated and can also contribute to excess urinary oxalate.
Measuring the oxalate content and determining the bioavailability of dietary oxalate present some technical problems. As a result, some dietary information may be misleading. Surprisingly, beverages such as tea or beer, thought to increase urinary oxalate excretion, may actually protect against stone disease.8,9 Finally, restriction of oxalate intake has been shown to reduce urinary oxalate levels, but not to prevent stone formation.
Calcium intake, particularly through dairy products, may be associated with hypercalciuria and stone formation. However, inverse relationships between dietary calcium and stone formation have been demonstrated, in that groups of men and women with the highest calcium intake have been shown to have nearly one half the rate of stones as groups with the lowest intake.10,11 One explanation for this phenomenon is that dietary calcium binds in the intestinal lumen with dietary oxalate, forming an insoluble, nonabsorbable complex. The reduction in urinary oxalate levels that occurs with increased intake of dietary calcium is proportionally more important than the increased urinary calcium levels. Like oxalate, some dietary calcium may also be less bioavailable.
LABORATORY EVALUATION
After a first episode of nephrolithiasis, a reasonable laboratory evaluation includes routine serum chemistries, including a blood urea nitrogen level, a creatinine level, electrolyte measurements and calcium, phosphate and uric acid levels. Children with stones should probably be referred to a urologist or nephrologist for further evaluation. Adult patients with a solitary kidney, struvite stones, abnormal renal function or renal tubular acidosis probably also require further evaluation.
Primary hyperparathyroidism, which is more common in women and older adults, should be considered in patients who have a high-normal or elevated serum calcium level (10 mg per dL or greater [0.25 mmol per L]). The preferred screening test for primary hyperparathyroidism is the intact parathyroid hormone level. High-normal serum calcium levels do not completely rule out this condition.12 Stone formation resulting from hypercalciuria may occasionally be a first presenting sign of sarcoidosis, often without hypercalcemia.
Urinalysis should be performed in patients with a first stone, and urine cultures should be obtained if infection is suspected. The presence of hexagonal cystine crystals may lead to the diagnosis of cystinuria. A low urinary pH is associated with uric acid stone formation, and a high urinary pH accompanied by a low serum bicarbonate concentration may occur with distal (type I) renal tubular acidosis. These patients are at risk for renal insufficiency. Calcium phosphate stones often occur in patients with renal tubular acidosis or hyperparathyroidism.
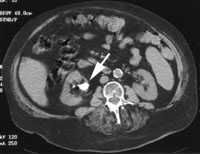
After the acute episode has resolved, imaging of the kidneys with ultrasonography or helical computed tomographic (CT) scanning is recommended to screen for conditions such as polycystic kidney disease or asymptomatic stones such as staghorn calculus (Figure 1). Unlike calcium stones, uric acid stones are radiolucent (unless mixed with calcium) and therefore are not visualized using plain radiographic techniques.13 These stones can be visualized directly on helical CT scans. Helical CT scanning is faster and more sensitive than intravenous pyelography (IVP), and it does not require the use of intravenous contrast material.14 It is more expensive than IVP, but the cost should decrease as the study becomes more widely available.
A 24-hour urine collection with measurement of relevant analytes is not generally indicated in most patients with a first stone. Generic therapeutic recommendations can be made without these data. Moreover, patients may not accept drug therapy after a single episode. A 24-hour urine collection may be warranted if a single stone was large, was associated with significant morbidity or occurred in an older patient more vulnerable to the adverse effects of acute urolithiasis and intervention.15 Cystinuria and primary hyperoxaluria, which are associated with more severe stone recurrences and more morbidity,16 should be ruled out in children with stones.
THERAPEUTIC RECOMMENDATIONS
After a first stone episode, up to 50 percent of patients have at least a second stone within 10 years, and up to 80 percent have more stones within 20 to 30 years.17 These data, combined with the memory of the pain, inconvenience and cost of the first acute stone episode, may motivate some patients to comply with suggested modifications in diet and fluid intake.
Treatment options are summarized in Table 3. The most important aspect of stone prevention is increased urine volume.8 Patients understand the concept that increased urine volume dilutes urinary constituents, and they are often able to achieve urine volumes of 2 to 2.5 L per day. This goal requires daily ingestion of 2.5 to 3 L of fluids to account for stool and insensible fluid losses. Fluid intake should be increased when perspiration is increased (e.g., with exercise and heat). Thirst is not a sufficient indicator of adequate hydration.
| Calcium stones | |
| 1. In all patient, increase fluid intake to yield an output of at least 2 L of urine per day. | |
| 2. In the patient with hypercalciuria: | |
| Dietary restriction of protein, oxalate and sodium; no restriction of dietary calcium | |
| Medication: thiazides, usually given with potassium citrate; amiloride (Midamor) | |
| 3. In the patient with hypocitraturia: | |
| Dietary restriction of protein and sodium | |
| Potassium citrate supplementation (sodium citrate if potassium citrate is not tolerated) | |
| 4. In the patient with hyperoxaluria: | |
| Dietary restriction of oxalate | |
| 5. In the patient with hyperuricosuria: | |
| Dietary restriction of purine (i.e., protein) | |
| Allopurinol (Zyloprim) | |
| Uric acid stones | |
| 1. Increasing fluid intake is less important for the prevention of uric acid stones than calcium stones. | |
| 2. In the patient with a low urinary pH level: | |
| Dietary restriction of protein and sodium | |
| Alkalinization of urine with potassium citrate (sodium citrate if potassium citrate is not tolerated) | |
| 3. In the patient with hyperuricosuria: | |
| Dietary restriction of protein and sodium | |
| Alkalinization of urine with potassium citrate if urinary pH level is low | |
| Allopurinol in selected situations | |
At least 8 to 12 oz of fluid should be ingested at bedtime, because urinary concentration usually occurs during sleep. Water is the preferred beverage. Citrus juices also appear to diminish the risk of stone formation, although the benefit of increased urinary citrate excretion is accompanied by a concomitant increase in urinary oxalate that may mitigate the net effect.18,19 Asking patients to periodically measure and record their own urine volumes using a soda bottle or a 24-hour collection jug may help to communicate the desired goal.
Although the effects of dietary changes on individual urinary components such as sodium and calcium are relatively well established, controlled trials demonstrating preventive efficacy are lacking. Restriction of daily salt intake to 2 g of sodium, animal protein intake to 8 oz and oxalate intake to as low as tolerated are all potentially useful, achievable goals. In particular, these restrictions are indicated if dietary excesses are uncovered in the history. Restriction of dairy products to reduce dietary calcium intake is no longer appropriate advice.
Patients may object to the combination of restricted intake of some vegetables (to lower urinary oxalate excretion) and animal protein (to lower urinary calcium and uric acid excretion). Similarly, patients already restricting saturated fat and beef intake for cardiovascular purposes may be disheartened to learn that dietary restrictions to prevent stones extend to fish, fowl and pork. Moderation, not elimination, should be the message.20
Recurrent Stone Formation
LABORATORY EVALUATION
Patients with recurrent stones should undergo a more detailed evaluation, including a 24-hour urine collection. Accurate diagnosis depends on the methods used to determine the relevant urinary electrolytes. The urine collection may be performed while patients follow their usual diet. Optimally, more than one collection should be made to account for day-to-day variability. To ensure proper handling, it is important to use a laboratory that specializes in the determination of lithogenicity (i.e., the likelihood of urinary stone formation).
The data obtained from analysis of the urine should include calculation of any supersaturation of relevant stone components. Supersaturation is a computed value that indicates the likelihood of crystallization given the concentration of relevant ions and the urinary pH. This value correlates well with stone composition and alleviation in response to treatment of the stone-forming state.21 It also allows the net effect of treatment and diet to be integrated into a single number that the physician and patient can use for long-term follow-up.
Hypercalciuria is the most common cause of calcium stones. Determining a specific cause of hypercalciuria using special diets and calcium binders is a technique best left to the research setting.22 Classifying patients with this technique is unwieldy and costly. Hypercalciuria can be treated in a nonspecific manner. Low-calcium diets and calcium binders such as sodium cellulose phosphate have no proven utility and may result in diminished bone density.
THERAPEUTIC RECOMMENDATIONS
One study found that the treatment of patients with recurrent stones is cost effective.23 The authors of this study calculated the costs of diagnostic testing, therapeutic procedures and medications in 1,092 unselected patients before and after metabolic evaluation and specific drug therapy. The drugs used, such as thiazides, potassium citrate and allopurinol (Zyloprim), are all relatively inexpensive. The savings achieved by the use of medical therapy and the resultant significant reduction in urologic procedures and hospitalizations amounted to $2,158 per patient per year. Given the proven benefit of prophylaxis, Medicare and most managed-care organizations typically cover the costs of diagnosis and these particular medical treatments.
The pharmacologic treatment of calcium stones requires lowering urinary calcium excretion with thiazides and increasing the inhibitory activity of urine by increasing urinary citrate excretion. Hydrochlorothiazide and chlorthalidone are useful for achieving lower urinary calcium levels,24 with the latter perhaps offering a more prolonged effect. The usual starting dosage of each agent is 25 to 50 mg once daily, with the dosage increased up to 50 mg twice daily as dictated by the results of repeated 24-hour urine collections.
The major side effect of thiazides is hypokalemia, which leads to reductions in urinary citrate excretion. Therefore, thiazide therapy is usually accompanied by potassium citrate supplementation to replace potassium and replenish urinary citrate. The need to restrict dietary salt intake to minimize kaliuresis and maximize the effect of reducing calciuria should be reemphasized. Potassium citrate comes in oral tablets and liquid forms (with various flavors to improve palatability). The usual dosage is 20 to 30 mEq taken twice daily as needed.
Another approach to hypokalemia is to add amiloride (Midamor), a potassium-sparing diuretic that may also have a minor effect in reducing urinary calcium excretion. Triamterene should not be used because it is poorly soluble and is associated with stone formation through precipitation of the drug.
Hypocitraturia is another major contributor to calcium stone disease.25 Potassium citrate supplementation can suffice to treat this condition. Potassium is the preferred citrate preparation because the sodium salt can increase urinary calcium excretion. Potassium citrate therapy may be limited by gastrointestinal intolerance, particularly in older patients and patients with dyspepsia. A recent formulation of magnesium and potassium citrate may minimize this side effect.26
Hyperuricosuria may also be an independent risk factor for calcium stones. In patients with this condition, allopurinol can be effective in reducing the ability of uric acid to facilitate the crystallization of calcium oxalate if other risk factors are not identified.27
Uric acid stones are most often appropriately treated with potassium citrate, which causes an increase in the urinary pH and, thereby, an increase in uric acid solubility. Allopurinol therapy is reserved for use in patients with hyperuricosuria, rather than low urinary pH level, as the dominant feature. Even in patients with hyperuricosuria, citrate therapy may be preferred, because excess uric acid can be solubilized at a more alkaline pH.7
Osteoporosis and Stone Disease
One potential issue is the significant incidence of osteoporosis in patients with stone disease and hypercalciuria.28 Mobilization of bone calcium may, in fact, be a primary cause of hypercalciuria.28 Many studies have shown that stone formers have lower bone density than nonstone formers matched by age and gender.29 Although the abnormality may be common, the bone disease usually appears to be mild, only rarely producing clinical signs.30 The syndrome may worsen with calcium restriction,29 which does not clearly prevent stone formation.
Bone density may increase with the administration of thiazides, which appear to have some beneficial effect on fracture rates.31 Bisphosphonates such as alendronate (Fosamax) may also be beneficial. The bisphosphonates, which are now standard therapy for osteoporosis, also reduce urinary calcium excretion.32 Therefore, therapy with thiazides and bisphosphonates may permit calcium supplementation in older stone formers who are at risk for osteoporosis.
Calcium citrate is the preferred agent for calcium supplementation in postmenopausal women with stone disease. Although urinary calcium excretion increases with the administration of this calcium salt, urinary citrate levels also increase.33 Supplementation using orange juice fortified with calcium citrate and calcium maleate is not associated with an increase in urinary calcium oxalate supersaturation.34