
Am Fam Physician. 2000;61(7):2131-2138
See related patient information handout about weight loss, written by the authors of this article.
Obesity is a common health problem in the United States, and effective treatment is challenging. Obesity is associated with an increased mortality rate and risk factors such as hypertension, hyperlipidemia and diabetes mellitus. Numerous treatments are available for obesity. Behavioral therapy, surgery and pharmacologic treatment have been used with varying degrees of success. Older anorectic agents have significant side effects and limited benefit, and some have even been withdrawn from the U.S. market because of a possible association with cardiovascular complications. The safety of newer agents must be extensively evaluated before widespread use is recommended. Therefore, behavioral therapy, including regular exercise and the development of healthy eating habits, continues to be the best treatment for long-term weight loss.
Obesity is one of the most common and serious health problems in the United States. Excess weight is independently associated with an increased mortality rate in multiple conditions (Table 1).1,2 Approximately one fourth of American adults (more than 60 million people) are overweight.2 Given this statistic, the Western cultural obsession with being thin and the societal and psychologic stigma of obesity, it is not surprising that, at any time, 50 percent of American women and 25 percent of American men are trying to lose weight, with an annual expenditure of $30 billion on weight loss treatments.2,3
| Type 2 diabetes mellitus* |
| Hypertension |
| Dyslipidemia |
| Macrovascular disease |
| Cancer (endometrial, ovarian, breast, gallbladder, prostate, colon) |
| Menstrual irregularities, decreased fertility, hirsutism |
| Gallbladder disease |
| Restrictive lung disease, sleep apnea |
| Osteoarthritis |
| Gout |
| Thromboembolic disease |
Etiology
In most persons, obesity is primary—no obvious cause exists other than an imbalance in energy intake and expenditure. Medical disorders such as Cushing's syndrome, hypothyroidism and hypogonadism rarely cause obesity. Genetic factors play a role, but the specific mechanism is unclear. Recently, a mutation in the gene coding for the beta3-adrenergic receptor has been found to be associated with an increased capacity to gain weight in some morbidly obese persons.4 In theory, low beta3-adrenergic activity could promote obesity by slowing lipolysis, causing retention of lipids in fat cells.5 Regardless of recent developments in understanding this problem, obesity should be considered a condition with multiple causes. Genetic, cultural, socioeconomic, behavioral and situational factors all play a role in dietary habits and weight control.
Evaluation
Assessment of the overweight or obese person should begin with a careful history and physical examination. The patient's weight history from childhood should be reviewed, including various methods of weight loss that have been attempted and the results of each attempt. Activity level and dietary history should also be reviewed. Because weight gain is a common side effect of certain medications (Table 2), a history of medication use is an important aspect of the initial evaluation.6 Weight gain is also common during the initial phases of smoking cessation.7
| Psychotropic agents | |
| Antidepresssant drugs (tricyclic antidepressants, monoamine oxidase inhibitors) | |
| Antipsychotic drugs | |
| Lithium | |
| Anticonvulsant agents | |
| Valproic acid (Depakene) | |
| Carbamazepine (Tegretol) | |
| Steroid hormones | |
| Corticosteroids | |
| Estrogen, progesterone, testosterone or other anabolic/androgenic steroids | |
| Insulin and most oral hypoglycemic agents | |
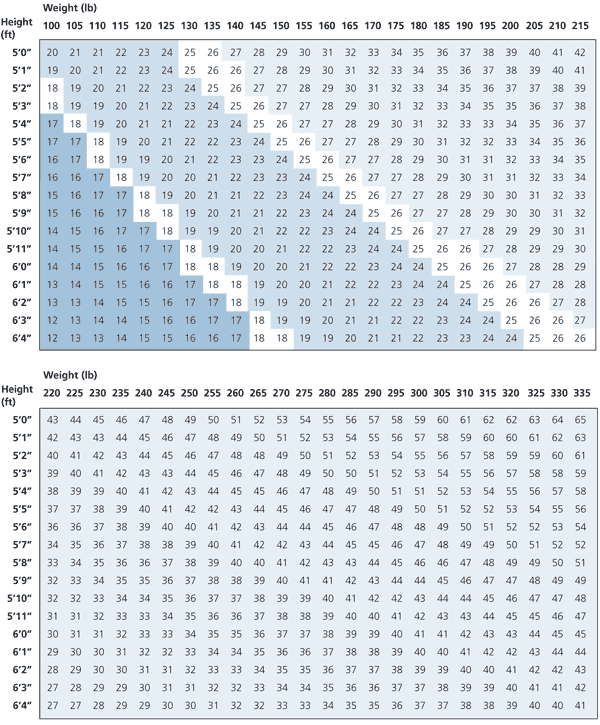
Body weight for height, gender and body-frame size has traditionally been used as the fundamental assessment of obesity. The National Institutes of Health and the National Heart, Lung, and Blood Institute recommend that all adults receive periodic measurement of height and weight by body mass index (BMI = weight in kilograms divided by height in meters squared) using standard tables (Figure 1)8 of suggested weights, along with the assessment of other factors such as medical conditions or waist-to-hip circumference ratio, as a basis for further evaluation, intervention or referral to specialists.2 “Overweight” is defined as a BMI of 25.0 to 29.9 kg per m2, and “obese” is defined as a BMI greater than 30 kg per m2.2 It may be useful to determine the distribution of body weight between fat and lean body mass in some patients attempting to lose weight; however, the effectiveness of these measurements in all patients is unknown.
Treatment
Like diabetes or hypertension, obesity is a chronic medical condition that is rarely cured; most often, the goal of treatment is palliation. Unfortunately, a safe and effective treatment for obesity that will satisfy most patients' desire for rapid and long-lasting weight loss is not available. A caloric deficit of 3,500 kcal is necessary to lose 0.45 kg (1 lb) of adipose tissue. Because most experts recommend losing no more than 0.45 to 0.90 kg (1 to 2 lb) per week, weight loss is typically slow, and recidivism is high.9
Of the many options for controlling obesity, behavioral therapy, including dietary modification, is preferable. In earlier studies, behavioral therapy was shown to be more favorable and cost-efficient than pharmacologic treatment for maintenance of weight loss.10 Although the use of pharmacotherapy produced a more rapid initial weight loss, the weight loss obtained using behavioral therapy was better maintained at one year. Physical activity and exercise are key to successful weight loss and weight loss maintenance.
Pharmacologic Therapy
APPETITE SUPPRESSANTS
Various pharmacologic agents, referred to as anorectic drugs, are used as adjuncts to behavioral therapy in weight reduction programs. The two classes of anorectic drugs currently available are the noradrenergic and the serotonergic agents.
Noradrenergic Agents. Noradrenergic drugs affect weight loss through action in the appetite center.11 Phenylpropanolamine (Dexatrim), a sympathomimetic drug and a synthetic derivative of ephedrine, is available as an over-the-counter appetite suppressant and decongestant. In studies lasting 14 weeks, the subjects who took phenylpropanolamine had a greater weight loss than those who took placebo, although the difference was minimal12,13 (Table 3).12–20 When taken in daily dosages of 20 to 75 mg, common adverse effects included nervousness, insomnia, dizziness, palpitations and headaches. Phenylpropanolamine in a dosage of 75 mg taken once daily was not associated with a clinically significant increase in blood pressure.13 When phenylpropanolamine is used in the treatment of obesity, the manufacturers recommend physician supervision if patients are also being treated for high blood pressure, depression or anxiety disorder, or if they have diabetes, heart disease or thyroid disease.11
| Agent | Daily dosage (mg) | Duration of therapy (weeks) | Mean weight loss, kg (lb) | ||
|---|---|---|---|---|---|
| Treatment group | Placebo group | ||||
| Phenylpropanolamine (Dexatrim)12,13 | 20 | 14 | 6.1 (13.4) | 4.3 (9.5) | |
| 75 | 14 | 5.96 (13.1) | 2.35 (5.2) | ||
| Phentermine (Ionamin)14 | 30.0 to 37.5 | 24 | 10.2 (22.4) | 4.4 (9.7) | |
| Fluoxetine (Prozac)*15,16 | 60 | 12 | 6.0 (13.2) | 6.7 (14.7) | |
| 60 | 52 | 1.7 (3.7) | 2.1 (4.6) | ||
| Sibutramine (Meridia)17,18 | 10, 15 | 24 | 5.8, 6.4 (12.8, 14.1) | 0.8 (1.8) | |
| 15 to 30 | 96 | 2.6 (5.7) | - | ||
| Orlistat (Xenical)19,20 | 120 three times daily | 52 | 9.5 (20.9) | 6.0 (13.2) | |
Phentermine (Ionamin) is structurally similar to amphetamine and modulates noradrenergic neurotransmission to decrease appetite; however, it has little or no effect on dopaminergic neurotransmission, which decreases its potential for abuse.11 The use of phentermine as a single agent is usually limited by an intolerance to its stimulatory activity. Phentermine was previously used in combination with fenfluramine (Pondimin) to improve weight loss and counteract the adverse effects of use of phentermine. Because of the withdrawal of fenfluramine from the U.S. market, phentermine is now used as a single weight-loss agent.
In older clinical trials, the use of phentermine alone resulted in significant weight loss when compared with placebo14 (Table 3). In dosages ranging from 30.0 to 37.5 mg per day, phentermine is labeled for the management of exogenous obesity as a short-term (i.e., a few weeks) adjunct in a regimen of weight reduction based on caloric restriction. The most common adverse effects of phentermine include headache, insomnia, nervousness and irritability. Palpitations, tachycardia and elevations in blood pressure may also occur. Phentermine should not be taken by persons with hyperthyroidism, glaucoma, agitated states, advanced arteriosclerosis, symptomatic cardiovascular disease, moderate to severe hypertension or a history of drug abuse.11
Serotonergic Agents. The serotonergic drugs partially inhibit the reuptake of serotonin and release serotonin into the synaptic cleft, thus acting on the hypothalamus to decrease satiety.11 Fenfluramine and dexfenfluramine (Redux), the first serotonergic agents labeled for the treatment of obesity, were withdrawn from the U.S. market in September 1997 because of case reports of valvular heart disease and primary pulmonary hypertension.21,22
Fluoxetine (Prozac) is a highly selective serotonin reuptake inhibitor (SSRI) that has been studied in the treatment of obesity.15,16 Fluoxetine may increase energy expenditure by raising basal body temperature; however, weight loss has not been consistent among subjects in clinical trials. In a three-month study, fluoxetine did not significantly reduce weight when compared with placebo15 (Table 3). In a longer clinical trial, significantly greater weight loss was achieved in the subjects taking fluoxetine at 20 weeks, compared with the subjects taking placebo. However, after one year, weight loss was not different in the two groups.16
Although fluoxetine has been labeled by the U.S. Food and Drug Administration (FDA) for the treatment of depression, bulimia and obsessive-compulsive disorder, the FDA has not labeled fluoxetine for weight loss therapy.
Adrenergic/Serotonergic Agents. Sibutramine (Meridia) is an adrenergic/serotonergic agent recently labeled by the FDA for use in the management of obesity.11 Sibutramine and its metabolite inhibit monoamine uptake, suppressing appetite in a fashion similar to SSRIs. Sibutramine may also stimulate thermogenesis by activating the beta3-system in brown adipose tissue. Initially tested for its antidepressant activity, sibutramine was found to cause weight loss 1 to 2 kg (2.2 to 4.4 lb) in healthy and depressed patients. In six-month studies, weight loss in subjects taking sibutramine, although modest, was found to be significantly greater than the loss in subjects taking placebo, and weight loss increased with increasing dosages17 (Table 3). In a continued, open-label, 96-week extension study, weight was regained even in subjects taking high-dose sibutramine.18
Sibutramine is indicated for the management of obesity, including weight loss and maintenance of weight loss, and should be used in conjunction with a reduced calorie diet. It is recommended for obese patients with an initial BMI of greater than 30 kg per m2, or greater than 27 kg per m2 in the presence of other risk factors (e.g., hypertension, diabetes, hyperlipidemia).
The recommended starting dosage of sibutramine is 10 mg administered once daily with or without food. If there is inadequate weight loss after four weeks, the dosage may be titrated to 15 mg administered once daily. The 5-mg dosage should be reserved for use in patients who do not tolerate the 10-mg dosage.11 The most common adverse effects associated with the use of sibutramine are dry mouth, anorexia, constipation and insomnia. A mild increase in blood pressure and heart rate have been noted in some nonhypertensive study participants.11
THERMOGENIC AGENTS
The combination of ephedrine and caffeine possesses anorectic and thermogenic properties with only mild, transient side effects. Ephedrine increases the release of norepinephrine, which modulates food intake and acts as a sympathomimetic agent to stimulate heart rate and blood pressure, and enhance thermogenesis. Caffeine, an adenosine antagonist, reduces the breakdown of norepinephrine within the synaptic junction. Ephedrine (20 mg) with caffeine (200 mg [combination product] or two to three cups of caffeinated coffee) taken three times daily was found to be more effective than placebo or either agent alone.23 Side effects from the use of an ephedrine/caffeine combination (tremor, insomnia and dizziness) were transient after eight weeks of treatment and comparable with placebo effects. This combination is not currently available on the U.S. market.
Selective beta3-adrenergic agonists are currently under investigation. They are believed to increase the rate of metabolism and cause a reduction in weight by decreasing the body lipid content.11 Interest in this class of drugs began with the recent discovery that a mutation in the gene coding for the beta3-adrenergic receptor was associated with weight gain, abdominal obesity and insulin resistance.4
DIGESTIVE INHIBITORS
Another strategy in the treatment of obesity is to use digestive inhibitors that interfere with the breakdown, digestion and absorption of dietary fat in the gastrointestinal tract. A reduction in fat is recommended in most weight loss diets; however, patient compliance with these diets is generally poor. Therefore, digestive inhibitors may have a role in creating the negative energy balance necessary for subsequent weight loss.
Gastric and pancreatic lipases aid in the digestion of dietary triglycerides by forming them into free fatty acids that are then absorbed at the brush border of the small intestine. Inhibition of these enzymes leads to inhibition of the digestion of dietary triglycerides and decreased cholesterol absorption, and may decrease absorption of lipid-soluble vitamins (A, D, E and K).24 Orlistat (Xenical), the first lipase inhibitor labeled by the FDA for treatment of obesity, is a potent and irreversible inhibitor of gastric and pancreatic lipases, preventing the absorption of about 30 percent of dietary fat.24,25
Orlistat is indicated for use in patients with a BMI of at least 30 kg per m2 or in patients with hypertension, diabetes or dyslipidemia who have a BMI of greater than 27 kg per m2.25 Some reports of the occurrence of breast neoplasm among users of orlistat delayed its initial release.26 Because orlistat has minimal systemic absorption, these findings were poorly understood and were investigated in randomized controlled trials. During these placebo-controlled studies, there was no difference in the incidence of breast cancer in patients taking orlistat versus patients taking a placebo.19,20
In double-blind, placebo controlled studies, weight loss during one year ranged from 3 to 4 kg (6.6 to 8.8 lb) with orlistat in a dosage of 120 mg three times daily versus placebo.19,20 Patients regained about one half as much weight (about 2 kg [4.4 lb]) during the second year of treatment with orlistat versus placebo. Statistically significant improvements in blood pressure, cholesterol levels, glucose and insulin measurements were noted in patients taking orlistat, but the difference was not clinically relevant.20
Gastrointestinal side effects occurred in as many as 40 percent of patients and resulted in discontinuation of use in about 10 percent of patients.19,20 Based on orlistat's mechanism of action, side effects would be more significant in patients eating a high-fat diet. Gastrointestinal side effects included flatus with discharge, oily spotting and oily stool, fecal urgency, fecal incontinence and abdominal pain. Lipid-soluble vitamin concentrations may change during therapy but rarely need supplementation. Orlistat does not appear to interfere with the efficacy of other chronically administered medications (i.e., antihypertensive agents, warfarin [Coumadin] and oral contraceptives).11
FAT SUBSTITUTES
In an effort to maintain the taste of foods while decreasing the fat content, American manufacturers have welcomed the development of fat substitutes. The goal of fat substitutes is to decrease caloric value from fat while maintaining the creaminess and richness derived from fat. The most recent fat-based substitute, olestra (Olean), contains zero kcal per g. Olestra is a sucrose polyester, labeled by the FDA for use as a food additive in prepackaged snacks (potato, corn and tortilla chips, and crackers) to replace 100 percent of the fat.27 As a sucrose polyester with six to eight fatty-acid side chains, it is too large to be hydrolyzed by digestive enzymes and, therefore, is not absorbed and has no caloric value. A 28-g serving of potato chips fried in fat contains 10 g of fat and 150 calories, while a similar serving of olestra potato chips contains no fat and only 70 calories.
The tolerability and safety of olestra have been studied extensively, but the effects on weight loss from long-term substitution of dietary fat with olestra have not been evaluated. A four-week study resulted in a 4-kg (8.8 lb) weight loss when olestra (in a dosage of 30 g per day) was substituted for dietary fat in a hypocaloric diet.28 Therefore, olestra may be effective in enhancing weight loss when used in a calorie-restricted diet.
The consumption of olestra-containing products has been shown to cause gastrointestinal side effects, such as bloating, flatulence, diarrhea, loose stools and anal leakage. In one study, participants were asked to consume a beverage and an unlabeled 369-g bag of potato chips made with olestra or potato chips made with triglycerides during a free movie screening.29 At two and 10 days after ingestion, there was no significant difference in the presence or frequency of gastrointestinal symptoms between the group ingesting olestra and the group ingesting triglycerides. However, significantly fewer chips containing olestra were consumed during the movie.
There is a concern that olestra may inhibit the absorption of fat-soluble vitamins (A, D, E and K) and carotenoids. It is important to remember that the absorption of fat-soluble vitamins from the digestive tract can only be affected by the presence of olestra if both foods are eaten at the same time. The FDA has required that all olestra-containing foods be supplemented (and so labeled) with vitamins A, D, E and K.20
The systemic bioavailability of lipophilic oral medications could potentially be altered by coadministration with olestra, but this has not been demonstrated in combination with propranolol (Inderal), diazepam (Valium), norethindrone (Aygestin), ethinyl estradiol (Estinyl) or norgestrel (Lo Ovral).30,31 Because of olestra's possible effect on vitamin K absorption, it should be used with caution in patients taking warfarin, although this effect has not been adequately studied.
HORMONAL MANIPULATION
The gastrointestinal tract and central nervous system contain several peptides and hormones that regulate feeding behavior. For example, cholecystokinin and serotonin act to decrease appetite and food intake.11 Conversely, neuropeptide Y increases food intake and decreases energy expenditure.11,32 Leptin may limit food intake, decrease plasma insulin and increase energy expenditure.11 Therefore, agonists and antagonists of these hormones and peptides are currently under investigation for the treatment of obesity.
Final Comment
If drug therapy is recommended in the management of obesity, it should be used in combination with a structured diet and an exercise program to achieve the greatest and longest lasting results. Because weight loss is difficult to maintain after discontinuation of drug therapy, the long-term impact of anti-obesity agents should be considered before initiation of pharmacotherapy. In addition, the safety of many of these agents has only been studied over a short period of time. Finally, the effect of weight loss obtained through the use of drug therapy on overall morbidity and mortality has not been established.