
Am Fam Physician. 2001;63(3):553-554
Migraine is a common and debilitating condition affecting approximately 18 percent of women during their reproductive years. Migraine episodes appear to be synchronized with the onset of menstruation in about 10 percent of women with the disorder, and there appears to be some debate about pathophysiologic differences between menstrual and other types of migraines. If the biochemical and other changes associated with menstrual migraine are unique, the response to conventional treatments may differ as well. Silberstein and colleagues conducted a retrospective review of two double-blind, randomized, placebo-controlled studies that evaluated the efficacy of rizatriptan, a 5 HT1B/1D receptor agonist, in treating menstrual migraine.
Data were analyzed from 1,413 women at 107 sites across the United States from two studies comparing the effectiveness of rizatriptan and placebo in treating menstrual migraine. Patient age, race and severity of headache were comparable in 534 women who took 10 mg of rizatriptan, 509 who took 5 mg and 376 who took a placebo. The proportions of women in each group who had a menstrual migraine were 26, 23 and 22 percent, respectively. The study compared reported symptom relief in each group of patients.
Both dosages of rizatriptan provided statistically significant pain relief at two hours compared with placebo. Photophobia and disability were eliminated as well (see accompanying figure). Use of escape medications was significantly reduced only in those taking the 10-mg dosage. No significant differences were found across groups in nausea relief when used for first attack. During subsequent migraine episodes, however, a statistically significant improvement was found in those taking the 10-mg dosage. These studies indicated that rizatriptan appears to be equally effective in providing symptom relief regardless of the cause of the migraine. Approximately 70 percent of patients reported pain relief two hours after ingestion of either dosage of this agent, compared with about 40 percent of women who had taken placebo. Headache recurred within 24 hours in 32 to 36 percent of patients taking rizatriptan, regardless of their menstrual status.
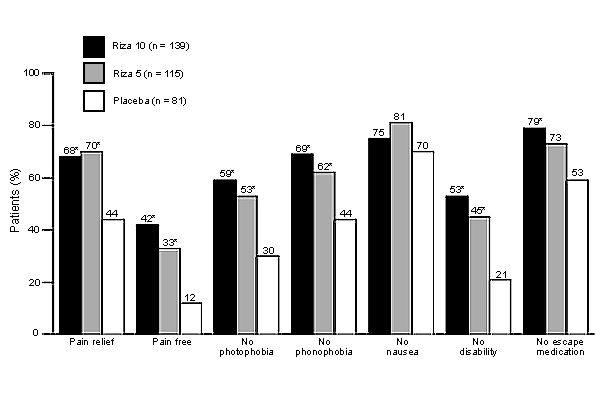
The authors conclude that both dosages of rizatriptan provided effective symptom relief regardless of whether the migraine was related to menstruation. This finding concurs with studies of sumatriptan, a related drug, which was also found to be equally effective in providing symptom relief for migraine headaches, regardless of cause.
editor's note: Results from these studies add to a growing body of evidence suggesting that menstrual migraines are not a distinct pathophysiologic condition, except that they are triggered by events in the menstrual cycle. The rates of symptom relief and the disturbing rates of recurrence are similar to those reported in other studies of triptans. Almost all of these studies have been supported by drug companies and compare triptans to placebo. In some patients, the 40 percent rate of pain relief at two hours with no recurrence that is obtained with placebo compares favorably with the 60 to 70 percent rate of pain relief at two hours with a 30 percent recurrence rate obtained using an expensive pill. A true comparison would be with symptomatic therapy. A European study comparing aspirin and metoclopramide with sumatriptan found comparable pain relief with less nausea and recurrence when aspirin and metoclopramide were given in adequate dosages.
There are few absolutes in migraine therapy. In some patients, the triptans are the most suitable pharmacologic agents; in others, they are expensive disappointments that prolong patients' “misery time.” Family physicians play an important role in helping patients and families obtain optimal control of this frustrating condition.—A.D.W.