Am Fam Physician. 2002;65(1):127-129
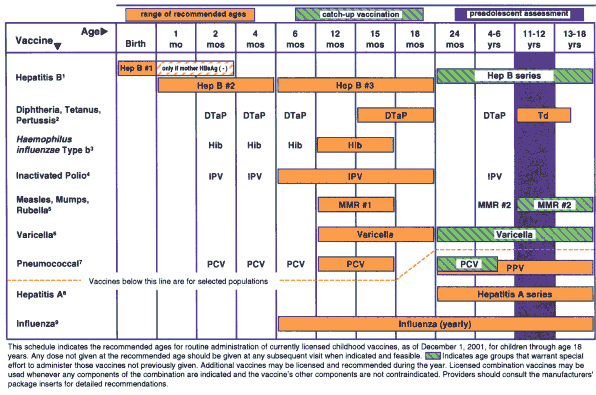
The format of the 2002 schedule differs somewhat from the 2001 schedule; it is based in large part on the modifications developed by the Minnesota Department of Health. First, the schedule uses color, which makes the recommended ages, catch-up ages, and the preadolescent status check stand out (see accompanying schedule on page 128). Second, vaccines for children at high risk because of underlying medical conditions or environment are included under the dashed red line. These include influenza, pneumococcal polysaccharide, and hepatitis A vaccines. The third change reflects the first dose of hepatitis B vaccine.
According to the Advisory Committee on Immunization Practices, the U.S. Preventive Services Task Force, and other authorities, all pregnant women should be screened for hepatitis B surface antigen (HBsAg). Infants born to HBsAg-positive mothers should receive hepatitis B vaccine and 0.5 mL hepatitis B immune globulin (HBIG) at separate sites within 12 hours of birth to achieve maximum protection against hepatitis B. However, proper maternal screening for HBsAg, transmittal of results to the physician caring for the neonate, and vaccination has not always been done, resulting in neonates unnecessarily contracting hepatitis B with fatal or potentially fatal consequences.1 Hence, the first dose of hepatitis B is shaded beyond birth to denote that it should be given at birth unless the mother's surface antigen status is known to be negative, in which case the range from birth to two months of age is acceptable.
After the first quarter of 2002 (i.e., March 31, 2002), use in children of hepatitis B, Haemophilus influenzae type b (Hib), and diphtheria and tetanus toxoids and acellular pertussis (DTaP) vaccines that contain thimerosal will no longer be recommended. There are several reasons for this decision. First, it is a way to lessen exposure to heavy metals. Second, it is a logical extension of previous recommendations for reduction of thimerosal in vaccines.2 Third, it responds to the report from the Institute of Medicine (IOM) that recommends vaccines without thimerosal be used for children in situations where an alternative is available, as is the case for hepatitis B, Hib, and DTaP vaccines.3 There have been no known harms associated with thimerosal-containing vaccines and the IOM found that the evidence is inadequate to accept or reject a causal relationship between thimerosal-containing vaccines and neurodevelopmental disorders.
I recommend that physicians check refrigerators for supplies of hepatitis B, Hib, and DTaP vaccines that contain thimerosal. Because immediate changes in policy could lead to delays in immunizing children and because a few children have unintentionally contracted vaccine-preventable diseases following quick changes in policy in the past, the new recommendation is being phased in over several months.1 Thus, thimerosal-containing vaccines may be used through March of 2002. Both manufacturers of hepatitis B vaccine will voluntarily replace thimerosal-containing hepatitis B vaccines. Manufacturers are no longer producing childhood hepatitis B, Hib, or DTaP vaccines with thimerosal (or the amount has been reduced to trace). Inactivated poliovirus (IPV); measles, mumps, rubella (MMR); varicella vaccine; and pneumococcal conjugate vaccine never contained thimerosal. Thimerosal-containing influenza vaccine may continue to be used as substantial amounts of an alternative are not available. Useful Web sites for current information includewww.immunizationed.org, a site developed by family physician educators;www.immunize.org;www.aafp.org;www.cdc.gov/nip; andwww.immunizationinfo.org.