
Am Fam Physician. 2002;66(5):823-829
Vaginal birth after cesarean section is common in this country. Physicians providing obstetric care should be aware of the potential complications. Uterine rupture occurs in approximately one of every 67 to 500 women (with one prior low-transverse incision) undergoing a trial of labor for vaginal birth after cesarean section. Rupture poses serious risks to mother and infant. There are no reliable predictors or unequivocal clinical manifestations of rupture, so physicians must maintain a high index of suspicion for possible rupture, especially in the presence of fetal bradycardia or other evidence of fetal distress. Management is surgery for prompt delivery of the infant and control of maternal hemorrhage. Newborns often require admission to an intensive care nursery. Prevention of poor outcomes depends on thorough anticipation and preparation. The physicians and the delivery institution should be prepared to provide emergency surgical and neonatal care in the event of uterine rupture.
Vaginal birth after cesarean section (VBAC) has become an integral part of modern obstetrics. With more than 100,000 VBACs achieved each year nationwide, this procedure may be viewed as a simple and routine method of delivery.1 However, experience has shown that VBAC is not risk free, and uterine rupture has been increasingly recognized as one of the complications that physicians should be ready to manage.1,2
Uterine rupture is a catastrophic tearing open of the uterus into the abdominal cavity. Its onset is often marked only by sudden fetal bradycardia, and treatment requires rapid surgical attention for good neonatal and maternal outcomes.
Avoiding the morbidity of repeat cesarean section through VBAC is a safe, attractive, and successful option in a majority of women.2–4 The purpose of this article is not to discourage or encourage VBAC, which would require a comparison of the relative risks of VBAC versus elective repeat cesarean. That analysis is outside the scope of this article, but it has been addressed elsewhere.5 Instead, this article focuses on an important complication of VBAC and encourages family physicians to maintain vigilance as VBAC is more widely implemented.1
Historical Perspective
In 1916, Cragin6 published a widely quoted recommendation, “Once a cesarean, always a cesarean.” His advice was probably influenced by the high rate of ruptures known to occur with the classic vertical incisions in use at that time.2,7–9 Cesarean sections using a safer, low-transverse uterine incision later became quite common.
In 1970, only 5 percent of all deliveries were cesarean, but this rate rose to 24.7 percent by 1988.2 Currently, approximately 1 million cesarean deliveries are performed each year.2,10 Promoting VBAC has been central to efforts to minimize surgical deliveries, contributing to a reduction in the rate of cesareans to 20.8 percent by 1995.2
Initial enthusiasm for VBAC has now been tempered by reports of poor maternal and fetal outcomes that can occur with failed attempts. It appears that the pendulum of consensus has swung from a restrictive approach to VBAC to active promotion and now back again to a position of caution.1 Accordingly, the American College of Obstetricians and Gynecologists (ACOG) has revised its guidelines for VBAC and now recommends a more careful approach.2
Incidence
True uterine rupture is typically distinguished from asymptomatic scar separation (dehiscence) by the need for emergency surgery, although some reports combine these separate processes and confuse the statistics.3,9,11–13 The rate of true uterine rupture with one prior low-transverse scar has been reported by ACOG to be between 0.2 and 1.5 percent (one of 67 to 500 women).2 Other studies involving more than 130,000 women undergoing a trial of labor for VBAC report rates that average 0.6 percent (approximately one of every 170 women).10,12–19
In women with two or more prior cesareans, the rate of rupture rises as high as 3.9 percent (one of 26 women).20 Such rates are threefold to fivefold higher than rates in women having only one prior cesarean delivery.10,21,22 A history of a successful prior vaginal delivery was found to reduce the risk of rupture from 1.1 to 0.2 percent (one of 511 women).20 Among less common incisions, classic and T-shaped uterine incisions are reported to rupture in 4 to 9 percent of cases, while low-vertical incisions carry a rupture risk of 1 to 7 percent.2 In comparison, rupture of an unscarred uterus occurs in one of 8,000 to 17,000 deliveries.3,23,24
Etiology
Many clinical conditions have been associated with uterine rupture.25,26 Table 12–4,7,11,15,21,24–29 outlines many of these factors. Labor is usually, but not always, required for uterine rupture. One third of ruptures in patients with a previous classic uterine incision occur before the onset of labor.7,9 Despite initial fears that epidural anesthesia would mask the pain of uterine rupture, recent evidence shows that use of this anesthesia during VBAC is safe.2,7,21 Amnioinfusion also appears to be safe and is not associated with an increase in rupture rates.18
| Uterine scars | |
| Prior cesarean section | |
| Prior rupture | |
| Trauma | |
| Injury from instrumentation during an abortion | |
| Significant myomectomy | |
| Any cause of uterine perforation | |
| Uterine anomalies (i.e., undeveloped uterine horn) | |
| Prior invasive molar pregnancy | |
| History of placenta percreta or increta | |
| Difficult forceps delivery | |
| Malpresentation | |
| Fetal anomaly | |
| Obstructed labor | |
| Induction of labor (suspected association) | |
| Excessive uterine stimulation | |
| Prostaglandin E1 (misoprostol [Cytotec]) | |
| Prostaglandin E2 (dinoprostone [Cervidil]) | |
| Oxytocin (Pitocin), especially high infusion rates | |
| Alkaloidal/crack-cocaine abuse | |
Excessive uterine stimulation can cause rupture, and this has occurred with alkaloidal cocaine abuse.27 Oxytocin (Pitocin) is widely used, so it is not surprising that this uterine stimulant has been administered in a majority of ruptures.7,24 One center found that oxytocin had been given in 77 percent of their ruptures and was typically used to stimulate labor in women with a prolonged latent phase.21
Misuse of oxytocin carries significant risks in any mother, and this risk may be increased during VBAC, especially at high infusion rates.2,11 ACOG guidelines and other authors indicate that oxytocin use during VBAC is acceptable.2,15,21 Induction of labor, regardless of the method used, is increasingly recognized as a risk factor for uterine rupture. Recent VBAC studies have shown three to five times more ruptures among induced mothers compared with those having spontaneous onset of labor.4,19 Experience with more potent uterine stimulants, such as prostaglandin E1 (misoprostol [Cytotec]) and prostaglandin E2 (dinoprostone [Cervidil]) continues to accumulate. While they were initially considered safe for use during VBAC, current reports describe ruptures in approximately 2.5 percent of women after their use (one out of 40 cases).2,4,11,19,28,29 Prostaglandin E2 appears to be weaker than prostaglandin E1 and yet has been found to cause 6.4 times more ruptures than a spontaneous trial of labor.4,19 Thus, these agents should be used with great caution during a trial of labor.
Diagnosis
Timely management of uterine rupture depends on prompt detection. In the past, caregivers were taught to look for classic signs such as sudden tearing uterine pain, vaginal hemorrhage, cessation of uterine contractions, and regression of the fetus.13,30 Recent experience has shown that these signs are unreliable and often absent.13 Instead, fetal distress has been found to be the most reliable presenting clinical symptom.13,15
Results of one study of 99 ruptures showed that only 13 patients reported pain and only 11 had vaginal bleeding.13 Prolonged, late, or variable decelerations and bradycardia seen on fetal heart rate monitoring are the most common—and often the only—manifestations of uterine rupture.13,15,17 Furthermore, uterine contraction patterns are unreliable for detecting rupture and often appear normal. Even ruptures monitored with an intrauterine pressure catheter (IUPC) often fail to show a loss of uterine tone or contractile pattern after uterine rupture.31–33
Shoulder dystocia related to fetal parts lodging outside the uterus can also be a presenting sign.34 Table 23,13,15,31–33 summarizes manifestations seen in several studies of reported rupture. Figure 133 shows a tracing from a published case of uterine rupture. It should be noted that it differs little from tracings that might be seen in other cases of fetal distress—uterine contractions continue (as measured by an IUPC), while fetal bradycardia develops.
| Manifestation | Incidence in selected reports (%) |
|---|---|
| Bradycardia as sole manifestation | 5 of 5 ruptures (100)15 |
| Bradycardia, fetal distress | 9 of 11 ruptures (82)3 |
| Abnormal fetal heart rate tracing | 23 of 70 ruptures (33)3 |
| Failure to progress | 15 of 70 ruptures (21)3 |
| Pain | 13 of 99 ruptures (13)13 |
| Vaginal bleeding | 11 of 99 ruptures (11)13 |
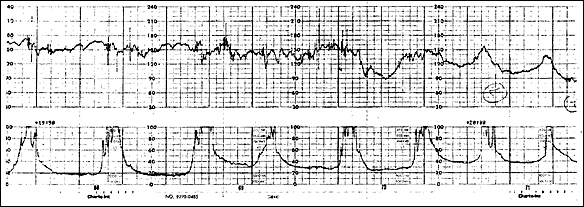
One author has concluded that “if a pro longed deceleration to 90 beats per minute or less lasting more than one minute occurs during a trial of labor, you should perform an immediate cesarean operation. Do not waste time performing an ultrasound examination or counting instruments. In many such cases, you will find no uterine rupture, but in other cases, you will have saved a baby's life.”1
Management
Because the presenting signs of uterine rupture are often nonspecific, the initial management of uterine rupture will be the same as that for other causes of acute fetal distress. Urgent delivery is indicated, which will typically mean a cesarean delivery. The physician should mobilize the hospital operating room team and, if necessary, call in the awaiting back-up surgeon. It is during surgery that a uterine rupture will be diagnosed and surgical correction initiated. On detection of this condition, the physician should ensure adequate intravenous access, arrange for sufficient blood transfusion, and call for a neonatal team to be ready for intensive-care newborn resuscitation. In one study, best outcomes were noted when surgical delivery was accomplished within 17 minutes from the onset of fetal distress on electronic fetal heart rate monitors.13
Complications
The life-threatening seriousness of uterine rupture is underscored by the fact that the maternal circulatory system delivers approximately 500 mL of blood to the term uterus every minute.25 Studies of ruptures have shown a loss exceeding 2,000 mL in one half of cases and a majority of women requiring blood replacement exceeding five units.15,23,30 Hysterectomy, with accompanying loss of future childbearing potential, has been required in 6 to 23 percent of cases to control maternal hemorrhage.13,30,35 Maternal death is a rare complication of rupture, though it is more common in ruptures occurring outside of a hospital and in women with an unscarred uterus.13,14,26 Overall, uterine rupture accounts for approximately 5 percent of all maternal deaths each year.26
Neonatal outcome after uterine rupture depends largely on the speed with which surgical rescue is carried out. Much of the published literature comes from large medical centers, where in-house physicians and support facilities are available for emergency surgery at any time.1,17 Even in such centers, newborn morbidity and mortality can be substantial. One large study's neonatal mortality rate was 2.6 percent, which rose to 6 percent when cases of rupture occurring before the mother reached a hospital were included.13 Older literature gives higher mortality rates of 13 to 100 percent, though many of the more recent studies report no fetal deaths at all.1,9,14,17,26 Outcomes seem to be worst when a fetus is extruded from the uterus into the peritoneal cavity,13,25,26 probably as a result of more extensive disruption of the maternal-placental circulation, which can lead to fetal asphyxia and potential long-term neurologic impairment.13,15 Although many infants delivered after uterine rupture do well, management often includes admission to a neonatal intensive care unit and, possibly, mechanical respiratory support.13,16
Prevention
Unfortunately uterine rupture cannot be adequately predicted among women desiring a trial of labor for VBAC, so constant preparedness is needed.13 Screening patients is helpful in some cases. In a patient with a known prior classic incision, repeat surgical delivery should be planned for before the point that spontaneous labor may be expected.7 Physicians also should review a woman's history for factors associated with higher rupture rates and give her a balanced understanding of her relative risks, benefits, alternatives, and probability of success. Helpful guidelines from ACOG are presented in Table 3.2 Signed documentation of this discussion and the patient's wishes should be placed in the medical record. A standardized consent form should be available from physicians' malpractice carriers, although some fear the legal language might drive patients away from appropriate VBACs.1
| Candidates for trial of labor |
| One or two prior low-transverse cesarean deliveries |
| Clinically adequate pelvis |
| No other uterine scars or previous rupture |
| Physician immediately available throughout active labor, capable of monitoring labor and performing emergency cesarean delivery |
| Availability of anesthesia and personnel for emergency cesarean delivery |
| Circumstances under which a trial of labor should not be attempted |
| Prior classic or T-shaped incision or other transfundal uterine surgery |
| Contracted pelvis |
| Medical or obstetric complication that precludes vaginal delivery |
| Inability to perform emergency cesarean delivery because of unavailable surgeon, anesthesia, sufficient staff, or facility |
Physicians are also advised to carefully review their hospital's resources for handling emergent complications such as uterine rupture.2 Guidelines published by ACOG indicate that trials of labor for VBAC should be carried out “in institutions equipped to respond to emergencies …,” and that there should be a “physician immediately available throughout active labor capable of monitoring labor and performing an emergency cesarean delivery.”2 This may make VBAC delivery in smaller hospitals problematic if blood banks, a surgeon, anesthesia, an operating room team, and neonatal support are not available at all times.
Many family physicians rely on consultation from others for cesarean deliveries, which may delay surgery in emergency cases. An important aspect of prevention is arranging for and confirming prompt surgical back-up before emergencies such as uterine rupture occur, or referring a patient to a center where more intense care can be provided.