
Am Fam Physician. 2004;70(12):2372-2375
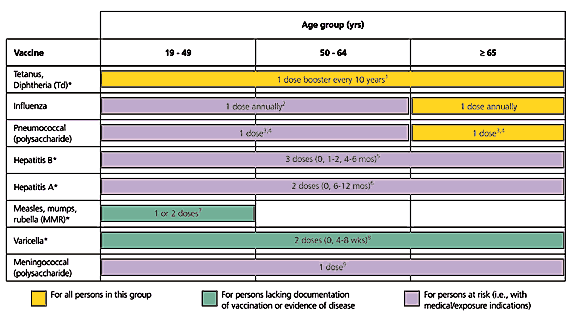
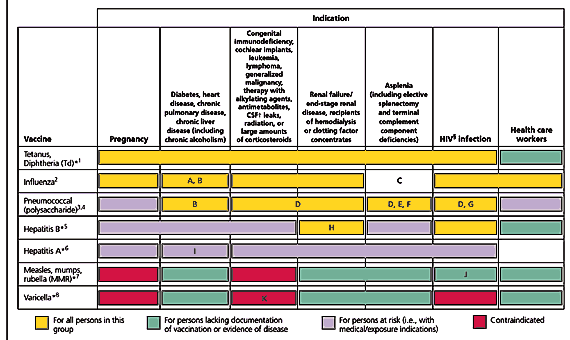
Cooperation continues between the American Academy of Family Physicians (AAFP), the Advisory Committee on Immunization Practices (ACIP), and the American College of Obstetricians and Gynecologists (ACOG) with the publication of the Recommended Adult Immunization Schedule by Age Group, Medical, and other Conditions, United States, October 2004–September 2005 (Figures 1 and 2). The age-based schedule is similar to the 2004 schedule, but there are two changes for medical conditions: (1) the addition of vaccines indicated for health care workers and (2) a change in color coding to differentiate vaccines when there is lack of documentation of immunity (green) and vaccines for persons at risk because of medical or exposure indications (lavender). The following examples may make this more clear.
Under the pregnancy column in Figure 2, influenza vaccine and tetanus and diphtheria (Td) boosters are indicated (as shown by the yellow bar). Hepatitis B vaccine is only indicated in women who are pregnant if they have a medical or exposure indication, such as sexually transmitted diseases or multiple sexual partners (as shown by the lavender bar). Measles, mumps, rubella (MMR), and varicella vaccines are contraindicated during pregnancy (as is shown in red).
Under the chronic disease column that includes diabetes and chronic pulmonary disease (Figure 2), influenza and pneumococcal polysaccharide vaccines are indicated (as shown in yellow). Because the influenza and pneumococcal mortality rates primarily are determined by the number of high-risk medical conditions, vaccination of high-risk persons is particularly important. Hepatitis A and B vaccines (as shown by the lavender bars) are only indicated for those with medical or exposure indications; footnote I (Figure 2) clarifies that all persons with chronic liver disease should be vaccinated against hepatitis A unless already immune. MMR and varicella vaccines are indicated if the person lacks documentation of previous vaccination or evidence of disease (as shown by the green bar).
Under the column for health care workers, vaccination is indicated against influenza (as is shown in yellow). Indeed, the best predictor of influenza attack rates in long-term care facilities is vaccination rates of the staff, not vaccination rates of patients. Pneumococcal polysaccharide vaccine is only indicated if the worker has a personal medical or exposure indication (as shown in lavender). Health care workers should have documented immunity or prior immunization to MMR, varicella, Td, and hepatitis B (as shown by the green bars and explained in detail in the footnotes on the last page of the schedule).
This fall, there has been a major shortage of inactivated influenza vaccine, as all clinicians are undoubtedly aware. The Centers for Disease Control and Prevention (CDC) is a good source of information about the shortage (http://www.cdc.gov/flu). One way to save inactivated influenza vaccine for high-risk groups is for health care workers and parents of infants less than six months of age to receive live, attenuated influenza vaccine (LAIV; FluMist). It is licensed for healthy persons five to 49 years of age. LAIV contains cold-adapted viruses that do not replicate well in the lower airways. LAIV can be given to health care workers as long as they are not in units with the most severely immunocompromised persons during the time that the patient needs reverse (protective) isolation (such as, bone marrow transplant unit); health care workers who are around persons infected with human immunodeficiency virus (HIV) and patients who are on dialysis can receive LAIV (see CDC publications1 for details). Although the optimal influenza vaccination season is October and November, influenza vaccine can be given December through March in persons who were not vaccinated during the fall.
Information on immunizations by family physician leaders for family physicians can be found at the Group on Immunization Education of the Society of Teachers of Family Medicine’s Web site athttp://www.immunizationed.org, which includes free handheld personal digital assistant software in both Palm and Windows formats. Materials for offices about adult immunization can be found at the National Partnership for Immunization’s Web site (http://www.partnersforimmunization.org), the Immunization Action Coalition Web site (http://www.immunize.org), the CDC National Immunization Program Web site (http://www.cdc.gov/nip), the National Coalition for Adult Immunization Web site (http://www.nfid.org/ncai), the National Network for Immunization Information Web site (http://www.immunizationinfo.org), and the AAFP Web site (https://www.aafp.org/x10615.xml).