
Am Fam Physician. 2005;71(7):1339-1346
The clinical evaluation of gastrointestinal bleeding depends on the hemodynamic status of the patient and the suspected source of the bleeding. Patients presenting with upper gastrointestinal or massive lower gastrointestinal bleeding, postural hypotension, or hemodynamic instability require inpatient stabilization and evaluation. The diagnostic tool of choice for all cases of upper gastrointestinal bleeding is esophagogastroduodenoscopy; for acute lower gastrointestinal bleeding, it is colonoscopy, or arteriography if the bleeding is too brisk. When bleeding cannot be identified and controlled, intraoperative enteroscopy or arteriography may help localize the bleeding source, facilitating segmental resection of the bowel. If no upper gastrointestinal or large bowel source of bleeding is identified, the small bowel can be investigated using a barium contrast upper gastrointestinal series with small bowel follow-through, enteroclysis, push enteroscopy, technetium-99m–tagged red blood cell scan, arteriography, or a Meckel’s scan. These tests may be used alone or in combination.
Although gastrointestinal bleeding is most commonly a result of benign anal pathology, life-threatening hemorrhage, cancers, and polyps must be considered in making the diagnosis.1 Acute, massive upper gastrointestinal bleeding has an incidence of 40 to 150 episodes per 100,000 persons annually, with a mortality rate of 6 to 10 percent.2–4 Acute, massive lower gastrointestinal bleeding has an incidence of 20 to 27 episodes per 100,000 persons annually, with a mortality rate of 4 to 10 percent.5,6 Mortality rates increase in patients with advancing age and increasing number of associated underlying comorbidities, specifically renal and hepatic dysfunction, heart disease, and malignancies.2–4,7
Gastrointestinal bleeding can present in several forms, depending on the rate of blood loss: microscopic blood loss presents as iron-deficiency anemia or hemoccult-positive stools; hematemesis is vomiting of fresh blood; “coffee-ground” emesis is vomiting of altered black blood; melena is black tarry stools; hemochezia is the passing of red blood via the rectum (usually from the lower gastrointestinal tract, but sometimes from a briskly bleeding upper gastrointestinal source).8
Most cases of gastrointestinal bleeding resolve spontaneously, regardless of the amount of blood lost.9–11 The stability of the patient and the rate of bleeding dictate the order in which various diagnostic procedures should be conducted. The goal is to identify and, if necessary, treat the source of bleeding, while maintaining hemodynamic stability.6,10
Evaluation
The evaluation of the upper or lower gastrointestinal tract for sources of gastrointestinal bleeding depends on whether the bleeding is acute massive hemorrhage or chronic intermittent bleeding10,12 (Tables 12–4,6,7,9,10,12–21 and27,13,22,23 ).Hospitalization is required in patients who are hemodynamically unstable or elderly, and those who have comorbidities. These patients usually are admitted in an intensive care setting, based on risk stratification criteria (Table 3).24 Patients with minimal or intermittent bleeding who are stratified as low risk can be evaluated in an outpatient setting.5,13
| Cause | Prevalence (%) | |
|---|---|---|
| Upper GI tract | ||
| Peptic ulcer disease | 40 to 79 | |
| Gastritis/duodenitis | 5 to 30 | |
| Esophageal varices | 6 to 21 | |
| Mallory-Weiss tear | 3 to 15 | |
| Esophagitis | 2 to 8 | |
| Gastric cancer | 2 to 3 | |
| Dieulafoy’s lesion* | <1 | |
| Gastric arteriovenous malformations | <1 | |
| Portal gastropathy | <1 | |
| Lower GI tract | ||
| Small bowel | ||
| Angiodysplasia | 70 to 80 | |
| Jejunoileal diverticula | — | |
| Meckel’s diverticulum | — | |
| Neoplasms/lymphomas (benign and malignant) | — | |
| Enteritis/Crohn’s disease | — | |
| Aortoduodenal fistula in patient with synthetic vascular graft | — | |
| Large bowel | ||
| Diverticular disease | 17 to 40 | |
| Arteriovenous malformations | 2 to 30 | |
| Colitis† | 9 to 21 | |
| Colonic neoplasms/post-polypectomy bleeding | 11 to 14 | |
| Anorectal causes‡ | 4 to 10 | |
| Colonic tuberculosis | — | |
| Variable* | 0 | 1 | 2 | 3 |
|---|---|---|---|---|
| Age (years) | < 60 | 60 to 79 | ≥ 80 | — |
| Shock | No shock; SBP ≥ 100 mm Hg; pulse < 100 bpm | Tachycardia; SBP ≥ 100 mm Hg; pulse ≥ 100 bpm | Hypotension; SBP < 100 mm Hg | — |
| Comorbidity | No major comorbidity | No major comorbidity | Heart failure, ischemic heart disease, or any major comorbidity | Renal failure, liver failure, or disseminated cancer |
| Diagnosis based on endoscopy | Mallory-Weiss tear, no lesion identified, and no SRH† | All other diagnoses | Malignancy of upper GI tract | — |
| Major SRH† | None or dark spot only | — | Blood in upper GI tract, adherent clot, visible or spurting vessel | — |
Acute Massive Rectal Bleeding
Acute massive rectal bleeding frequently arises from an upper gastrointestinal source2,10,16,25 (Table 12–4,6,7,9,10,12–21). When there is evidence or clinical suspicion of an upper gastrointestinal source of bleeding, the diagnostic work-up begins with an esophagogastroduodenoscopy (EGD), which is the diagnostic tool of choice for evaluation of lesions above the ligament of Treitz.6,9,10,16,26,27 Table 43,4,10,21,28–30 describes the history and clinical findings associated with gastrointestinal sources of rectal bleeding. If the patient is not experiencing hematemesis and endoscopy is not immediately available, a nasogastric tube may be placed for gastric lavage while awaiting endoscopy.10,12 If no blood is returned and bile is identified, an upper gastrointestinal source is much less likely, and the work-up can focus on the large bowel.6,9,10,12,13
| Cause | History and clinical findings | |
|---|---|---|
| Upper GI tract | ||
| Peptic ulcer disease | Use of aspirin, NSAIDs, or tobacco | |
| Esophageal varices | Alcohol abuse; jaundice; signs of portal hypertension, including: ascites, palmar erythema, spider angiomata, hepatomegaly, splenomegaly, and rectal varices | |
| Mallory-Weiss tear | Bleeding preceded by vomiting, retching, or seizures | |
| Gastric cancer | Left supraclavicular adenopathy; palpable mass; abdominal pain; weight loss; cachexia | |
| Lower GI tract | ||
| Diverticular disease | Age > 60 years; painless bleeding; possible recent constipation | |
| Arteriovenous malformations | Age > 60 years; painless bleeding; chronic renal failure | |
| Colonic neoplasms | Age > 50 years; abdominal pain; weight loss; muscle wasting; protein calorie malnutrition; right-sided colon cancer may be associated with palpable right-sided abdominal mass; hepatomegaly; liver nodules; history of adenomatous polyps or longstanding ulcerative colitis; prior exposure to ionized radiation; family history of familial polyposis coli or cancer family syndrome | |
| Inflammatory bowel disease | Ulcerative colitis: starts in younger patients (20 to 40 years of age); usually involves the rectum; associated with diarrhea mixed with blood and mucus | |
| Crohn’s disease: starts in younger patients (20 to 40 years of age); perianal, peritoneal, and/or abdominal wall fistulas may be associated | ||
| Radiation colitis | History of radiation treatment to abdomen and/or pelvis | |
| Hemorrhoids | Perianal mass may be painful (external hemorrhoid) or painless (internal hemorrhoid); commonly starts in younger patients; associated with constipation, pregnancy, or postpartum period | |
| Anal fissures | More common in patients with history of constipation; associated with severe sharp pain occurring with straining on defecation; pain resolves within an hour after defecation; commonly starts at 20 to 40 years of age | |
| Colon tuberculosis | History of pulmonary tuberculosis or past exposure to tuberculosis | |
| Aortoduodenal fistula | History of abdominal aortic aneurysm surgically repaired with synthetic vascular graft placement | |
Colonoscopy is one of two diagnostic tools of choice used to evaluate acute lower gastrointestinal bleeding6,10,12,13,26 (Table 12–4,6,7,9,10,12–21). Several studies have demonstrated that colonoscopy identifies definitive bleeding sites in more than 70 percent of patients.2,6,10,12,16,31,32 Colonoscopy may be performed urgently or electively, depending on the patient’s hemodynamic status and risk-stratification criteria.
If bleeding stops or hemodynamic stability is achieved, colonic preparation may precede colonoscopy to increase visibility and diagnostic yield.31 Advantages of colonoscopy include direct visualization; access for tissue biopsy; and the ability to treat bleeding lesions primarily with heat probe, epinephrine injection, laser therapy, band ligation, or hemoclipping.12,14,16 As an initial diagnostic test, colonoscopy has a higher yield and a lower complication rate than arteriography.6,12,13 When used to evaluate sub-massive lower gastrointestinal bleeding, colonoscopy is highly effective; however, in cases of massive hemorrhage it may be limited by poor visibility.6,12
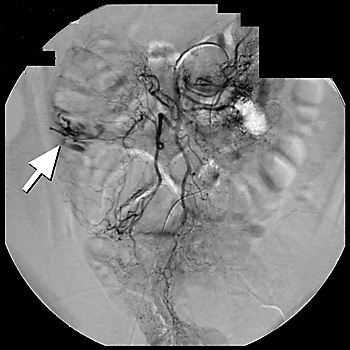
Arteriography is a radiographic contrast study that can identify briskly bleeding sources.11,16,22 In situations where massive bleeding impedes visualization of the colon, arteriography may be used as a second-line diagnostic tool of choice.6,10,12,13,16 Although several studies have shown a broad range of overall sensitivity with arteriography (40 to 78 percent), the largest study6 reported a diagnostic sensitivity of 41 percent.6 Mesenteric arteriography can identify bleeding from arteriovenous malformations by demonstrating extravasation of contrast material into the bowel lumen, which helps localize the bleeding site16,24 (Figure 1).
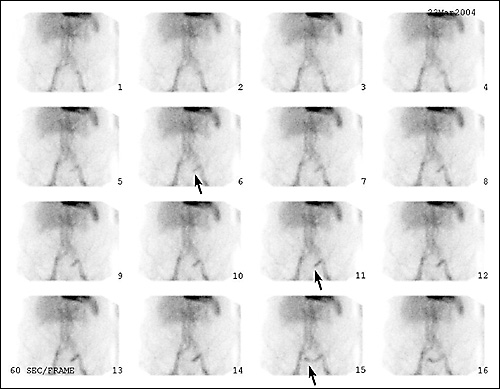
A technetium-99m–tagged red blood cell scan is a nuclear study best suited for identifying slow-bleeding sources with rates of 0.1 to 0.4 mL per minute.6,11,12,16,22 However, this test is not as accurate as arteriography in identifying the exact location of a bleeding site. When arteriography is used in association with a technetium-99m–tagged red blood cell blush, the sensitivity of the arteriogram is increased to 61 to 72 percent.22 One five-year, retrospective study33 of technetium-99m–tagged red blood cell scans showed that an “immediate blush” (positive scan) had a 60 percent positive predictive value for an associated positive angiogram. A “delayed blush” correlated with a predictive value of 93 percent for a negative angiogram. This finding suggests that a positive “immediate blush” is a good indication for urgent angiography or surgery, while a delayed blush or negative technetium-99m–tagged red blood cell scan is an indication for observation and elective colonoscopy33,34 (Figure 2).
When the source of bleeding cannot be identified by endoscopic, radiographic, or nuclear intervention, the patient may need exploratory laparotomy.10,12,13,16,22 In most circumstances this is accompanied by intraoperative endoscopy,22 which has a sensitivity of more than 70 percent for identifying sources of bleeding and limits the extent of surgery in up to 10 percent of cases.6,22,37 However, the removal of identified colon lesions does not always result in effective treatment of the underlying source of bleeding.22 In these cases, arteriography can be used intraoperatively as an adjunct to localize a source of bleeding, facilitate segmental resection of the bowel, and prevent blind hemicolectomy.22
When rectal bleeding stops before the source is identified, evaluation can proceed in the outpatient setting in patients who remain stable and at low risk.5,13 Depending on clinical suspicion, it may be appropriate to repeat upper or lower endoscopy, because upper and lower gastrointestinal lesions occasionally are missed on the first endoscopic evaluation.22 The most commonly missed upper gastrointestinal lesions are erosions in large hiatal hernias, arteriovenous malformations, and peptic ulcers.22 The lower gastrointestinal lesions most commonly missed on initial endoscopy are arteriovenous malformations and neoplasms.22
Chronic Intermittent Rectal Bleeding
EGD is the diagnostic test of choice for suspected chronic intermittent rectal bleeding9,22 (Table 27,13,22,23). A barium-contrast upper gastrointestinal series with small bowel follow-through (SBFT) may be considered if there is a relative contraindication to endoscopy (i.e., patient preference, concomitant anticoagulation treatment, comorbidity causing unacceptable risk for conscious sedation, or unavailability of an endoscopist).22 This procedure has a sensitivity of 54 percent and a specificity of 91 percent in the detection of upper gastrointestinal lesions located above the ligament of Treitz. Upper endoscopy has a 92 percent sensitivity and a 100 percent specificity.22
Colonoscopy is the diagnostic tool of choice in a hemodynamically stable patient with a suspected lower gastrointestinal source of bleeding.10,12,22,23,38 One prospective study39 showed that colonoscopy had a higher positive predictive value than double-contrast barium enema with sigmoidoscopy in identifying all colonic lesions (87 versus 81 percent, respectively). Colonoscopy also has a higher sensitivity than double-contrast barium enema in identifying colon cancer (97.5 versus 83 percent, respectively), neoplastic polyps greater than 1 cm (91.4 versus 21.7 percent, respectively),38 and angiodysplasia. Most importantly, colonoscopy allows for evaluation of the entire colon while providing the opportunity to acquire tissue biopsy and facilitating therapeutic intervention.39
Some physicians may consider a limited evaluation of the anorectosigmoid area in patients younger than 40 years because only 5 percent of colorectal cancer cases occur in this population.40 In addition, the most common cause of rectal bleeding in patients younger than 30 years is anal pathology such as hemorrhoids or fissures.1 In cases where the source of bleeding cannot be confirmed, or if bleeding and anemia continue, a colonoscopy should be performed for complete evaluation of the large bowel.41
Small Bowel Bleeding
Small bowel sources of gastrointestinal bleeding are uncommon, accounting for only 2 to 10 percent of all cases. Because of its location, evaluation is technically difficult. For these reasons, therefore, evaluation of the small bowel is less commonly indicated.16,42 However, when endoscopy is nondiagnostic, the small bowel should be evaluated. When evaluation of the small bowel is considered necessary, several procedures can be employed.22,42,43
One diagnostic tool is push enteroscopy, which is an extension of upper endoscopy that allows visualization of 15 to 160 cm of small bowel distal to the ligament of Treitz.6,22,42 This procedure allows for tissue biopsy and treatment of bleeding lesions, but it is limited by its inability to visualize beyond 160 cm of the proximal small bowel.22,42 The diagnostic yield for push enteroscopy is approximately 54 percent.22
Two radiographic tools are used to evaluate the small bowel. One is a barium-contrast upper gastrointestinal series with SBFT, which has a low sensitivity (zero to 5.6 percent).22 The other diagnostic tool is enteroclysis, which has a sensitivity of 10 to 21 percent.22 The latter procedure requires a small tube or endoscopic placement of contrast material directly into the proximal small bowel.22 The advantages of enteroclysis over SBFT are its higher sensitivity, shorter procedure time, and greater usefulness in evaluating an unconscious or uncooperative patient.22 When enteroclysis is combined with push enteroscopy, the absolute yield over push enteroscopy alone increases from 54 to 58 percent.22
One of the nuclear studies routinely used in the evaluation of the small bowel is technetium-99m–tagged red blood cell scanning.22,42 The other is a Meckel’s scan, which uses technetium-99m pertechnate.22,42 A Meckel’s scan has a high sensitivity (75 to 100 percent) for identifying gastric mucosa in the small bowel, but cannot confirm the identified lesion as the source of bleeding.22 This study is most appropriately used in the evaluation of younger patients.6,22
Although arteriography is most useful in the evaluation of acute massive rectal bleeding, it also serves a role in the evaluation of obscure sources of bleeding that are not identified by endoscopy.16 It is particularly helpful in the evaluation of older patients in whom arteriovenous malformations or neoplasms are suspected, because both of these lesions are associated with characteristic vascular patterns that can be identified on arteriogram.16 Helical computed tomography combined with angiography also may have a role in evaluating obscure sources of bleeding. More evidence-based studies need to be done to confirm the latter as an option.44
Capsule endoscopy is a new diagnostic tool consisting of a pill-shaped camera that the patient swallows42 (Figure 3). Only a few small studies have examined the usefulness of this diagnostic tool in the evaluation of sources of small bowel bleeding, but the preliminary results are promising.42,43,45,46 When compared with push enteroscopy (38 percent yield), capsule endoscopy has been shown to have the better diagnostic yield (66 to 69 percent) in identifying small bowel lesions.34,42,43
A recent prospective study45 comparing capsule endoscopy with barium-contrast upper gastrointestinal series with SBFT found capsule endoscopy to have a superior diagnostic yield in identifying obscure bleeding sites (31 versus 5 percent, respectively). Capsule endoscopy is painless and well tolerated, and requires no sedation.34,43 Although capsule endoscopy is helpful in identifying pathologic lesions, it must be followed by endoscopy or surgery for tissue diagnosis and treatment of significant sources of bleeding.42 Capsule endoscopy is contraindicated in patients when bowel stricture is suspected.34,42,46
When all diagnostic tools have failed to allow identification or effective treatment of chronic recurrent bleeding, and anemia persists or worsens, laparotomy with intraoperative enteroscopy may be indicated.37 This procedure is considered a last option in the diagnostic evaluation of nonemergent cases because it is more invasive and associated with higher rates of morbidity and mortality.22