
Am Fam Physician. 2008;78(11):1254-1262
Patient information: See related handout on radiation therapy, written by the authors of this article.
Author disclosure: Nothing to disclose.
Recent advances have improved the effectiveness, decreased the complications, and expanded the implications of radiation therapy. These advances include three-dimensional conformal radiation therapy, intensity-modulated radiation therapy, stereotactic radiotherapy, brachytherapy, and radioimmunotherapy. Each of these modalities has improved radiation targeting, thereby limiting radiation exposure of healthy tissues. The way radiation therapy is administered has also changed. Although traditional external beam radiation therapy is administered daily over several weeks, stereotactic radiotherapy may be administered as a one-time treatment. Radioimmunotherapy is administered intravenously. Contemporary radiation techniques also have distinct toxicity profiles. The high radiation doses employed during stereotactic radiotherapy have been associated with obliteration or obstruction of tubular structures, such as bronchi and bile ducts, limiting its use near these tissues. Radioimmunotherapy may be complicated by anaphylactic reactions during and following infusions. As more patients are diagnosed with cancer and as these patients live longer, primary care physicians will increasingly care for those who have received radiation therapy.
Radiation therapy has a pivotal role in the treatment of cancer. Indications for radiation therapy (Table 1) range from definitive treatment of localized tumors to palliation of symptoms from widely metastatic disease. In certain circumstances, radiation therapy has disease control rates comparable with those of surgery, but with less morbidity. For instance, many advanced laryngeal cancers are treated with radiation and chemotherapy instead of with laryngectomy, allowing most patients to retain their voice after treatment.1,2 In some patients, radiation therapy can be used before surgery, allowing for a more limited, safer, and more effective surgery. Since the 1980s, the use of regional radiation after local excision (lumpectomy) of early-stage breast cancer has spared thousands of women the morbidity and disfigurement of a mastectomy.3–5
| Clinical recommendation | Evidence rating | References |
|---|---|---|
| Radiation therapy should not be administered during any trimester of pregnancy. | C* | 5 |
| Initial use of a plain, nonscented, lanolin-free hydrophilic cream is helpful for patients experiencing radiation skin reactions. | C | 41 |
| Amifostine (Ethyol) may be considered to decrease the incidence of xerostomia in certain patients undergoing fractionated radiation therapy to the head and neck region. | B | 38 |
| For most women with stage I and II breast cancer, breast-conserving therapy with lumpectomy, axillary lymph node dissection, and whole breast radiation therapy is equivalent to mastectomy withaxillary lymph node dissection. | A | 4, 5 |
| Dental examination and treatment are important before starting radiation therapy, especially for patients with head and neck cancer, and should continue throughout treatment and follow-up. | C | 39 |
| Patients with low-risk prostate cancer may be treated with radiation therapy or surgery. | B | 30, 31 |
| Application of therapy | Principle | Common examples | |
|---|---|---|---|
| Primary (all patients) | Compared with surgery, radiation offers improved or equivalent tumor control with less morbidity | Anal cancer, head and neck cancer (e.g., laryngeal, oropharyngeal) | |
| Outcomes and toxicities are similar between radiation and surgery; therefore, both require an individualized assessment and discussion of the patient's condition and preferences | Cervical and prostate cancers, acoustic neuroma, meningioma | ||
| Patients medically unfit for surgery | Cardiac, pulmonary, or other chronic disease precludes surgery, but not radiation therapy | Endometrial and lung cancers | |
| Anatomically unresectable cancers | Close proximity to critical structures (e.g., blood vessels) precludes surgery, but not radiation therapy | Bladder, pancreatic, and skin cancers | |
| Preoperative | Shrinks the tumor, facilitating subsequent surgical resection | Esophageal and rectal cancers | |
| Postoperative | Decreases risk of local or regional tumor recurrence; treats areas with a known tumor if there is gross residual disease or positive surgical margins after resection | Breast, endometrial, gastric, pancreatic, and rectal cancers; malignant glioma; sarcoma; seminoma | |
| Palliative | Relieves bony pain | Breast, lung, prostate, renal, other cancers that are metastatic to bone | |
| Stops or limits bleeding | Gastrointestinal, genitourinary, and lung cancers | ||
| Relieves luminal (airway, biliary, gastrointestinal) obstruction | Lung and colon cancers | ||
The principal limitation of radiation therapy is radiation exposure of healthy tissues. Radiation toxicities, such as cognitive dysfunction, esophagitis, and myelosuppression, depend on the irradiated organs and on the radiation dose and scheduling. Traditionally, radiation oncologists have limited adverse effects by reducing the dose of radiation or by spreading the dose over multiple administrations (i.e., dose fractionation). Over the past decade, advances in radiation planning and delivery have markedly improved the ability to focus radiation on target tissues, sparing nearby healthy tissues. Table 2 summarizes modern radiation therapy modalities.
| Modality | Description | Indications/uses | Administration |
|---|---|---|---|
| External beam radiation therapy | |||
| Three-dimensional conformal radiation therapy | CT or MRI is used to target tumors while minimizing radiation exposure of healthy tissues | Most solid tumors | Daily outpatient treatments (as short as one to two minutes each), administered Monday through Friday for two to seven weeks; overlying skin may be marked with freckle-size tattoos or colored ink marks to guide the radiation beam; a mesh face mask or body mold may be used to immobilize the patient |
| Four-dimensional radiation therapy | Computer-assisted tracking or gating of CT images of moving targets | Tumors that are susceptible to movement, most commonly in the lung, liver, pancreas, or breast | Similar to three-dimensional conformal therapy; for gating, patients may be asked to hold their breath while the radiation beam is activated |
| Intensity-modulated radiation therapy | The radiation beam is divided into components (“beamlets”), which permits sparing of normal tissues | Tumors surrounding or adjacent to normal critical structures, most commonly head and neck or prostate cancers | Similar to three-dimensional conformal therapy, although individual treatments may last more than 30 minutes |
| Stereotactic radiosurgery (e.g., Gamma Knife) | Multiple radiation beams converge on target tumor, delivering high-dose radiation to the tumor, but little to surrounding tissues | Intracranial lesions, such as brain metastases, meningiomas, acoustic neuromas, arteriovenous malformations, and trigeminal neuralgia | Single treatment; to ensure proper patient positioning and immobility, a positioning frame is secured to the patient's skull, then attached to the radiation source; treatment lasts 45 to 60 minutes |
| Stereotactic body radiation therapy (e.g., Cyberknife) | High-dose radiation delivered using robotic guidance | Treatment of spine tumors, localized lung cancer, and other tumors in patients who are not candidates for surgery | Most commonly delivered as three to five fractions; during treatment, a robotic arm containing the radiation source (a linear accelerator) rotates around the patient to deliver radiation from multiple positions; each treatment lasts up to two hours; positioning may be accomplished using fiducial markers placed beforehand or using a rigid body frame |
| Internal radiation therapy | |||
| Temporary brachytherapy implant | A radiation source is placed within or near the tumor target and is subsequently removed | Cervical cancer, sarcoma, vaginal cancer, oral cavity cancers | Catheters (smaller) or applicators (larger) are placed in body cavities or tissues; subsequently, the radiation source is placed within these devices; the patient may be hospitalized in a private room during treatment (radiation source is left in place throughout treatment), or the patient may undergo outpatient treatment for up to several weeks (radiation source is removed between treatments) |
| Permanent brachytherapy implant | A low-dose rate (i.e., long half-life) radiation source is placed within or near the tumor target | Prostate cancer | Radioactive seed implants are inserted into target tissue through a catheter under local or general anesthesia; initially, the patient may be required to limit social contacts after placement for up to one month; implants are never removed, but radiation dissipates within six months |
| Systemic radiation therapy | Systemically administered radioisotopes target tumor cells | Iodine-131 for thyroid cancer; strontium-89 and samarium-153 for painful bony metastases; yttrium-90 ibritumomab tiuxetan (Zevalin) and iodine-131 tositumomab (Bexxar) for non-Hodgkin lymphoma | Administered intravenously or orally; inpatient or outpatient, depending on specific treatment; patients are required to follow radiation precautions (careful disposal of body fluids, including urine, sweat, and tears; hand washing; condom use) for one week after treatment |
Radiation Principles and Modalities
The underlying principle of radiation therapy is the destruction of malignant tissues while minimizing damage to normal tissues within a treatment field. Ideally, this would be accomplished by avoiding normal tissues altogether, but the relatively crude targeting of traditional radiation techniques does not permit such precision. Instead, a therapeutic ratio is achieved by making use of the different radiosensitivities of normal tissues and tumors. Ionizing radiation kills cells by causing DNA strands to break and cross-link. In general, normal cells are better able to repair this damage to DNA than are cancer cells. Administering relatively small daily doses of radiation over several weeks permits healthy cells to recover between sessions, while causing cumulative damage to tumor cells. Dose fractionation has been the central paradigm of treatment delivery in radiation oncology.6
External beam radiation therapy, or teletherapy, accounts for almost 90 percent of radiation treatments. This technique involves the delivery of electromagnetic radiation (e.g., x-rays, gamma rays) or particulate radiation (e.g., electrons, protons) from a linear accelerator or radionuclide source, such as cobalt-60. Alternatively, in brachytherapy, a radiation supply—usually contained in seeds, rods, or liquid—is placed within the patient.
Shortcomings of Traditional Radiation Therapy
Until the 1980s, radiation oncologists devised treatment plans using plain radiography, which rarely visualized a tumor directly. This treatment approach was associated with uncertainties, inconveniences, and toxicities. Because only an approximate location of the cancer could be determined, the radiation field needed to include a generous margin. For example, in prostate cancer therapy, the treatment volume usually included portions of the gastrointestinal and genitourinary tracts. This led to radiation proctitis (characterized by fecal urgency and rectal pain and bleeding) in up to 40 percent of patients7; sexual dysfunction in up to 50 percent of patients8; and urinary complications (e.g., incontinence, hematuria, strictures) in up to 10 percent of patients.9 Because radiation was typically administered over 30 or more daily fractions (fractional doses), the location of the target tumor varied throughout treatment. Slight changes in patient position were inevitable, and shifting rectal contents altered the prostate's anatomic position. In some patients, such organ movement led to underdosing of the target tumor and increased relapse rates.10
Modern Radiation Therapy Techniques
EXTERNAL BEAM RADIATION THERAPY
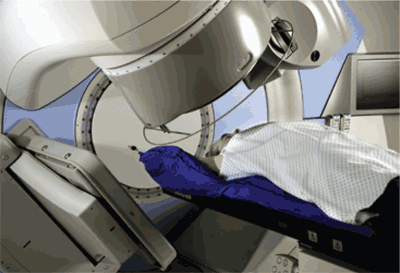
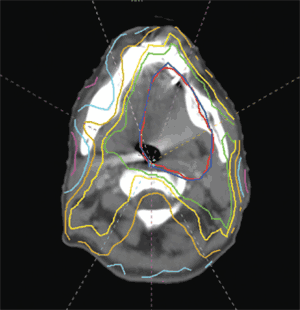
A series of incremental technologic advances has improved the targeting of external beam radiation therapy. Computed tomography (CT) and magnetic resonance imaging (MRI) have largely replaced plain radiography in radiation treatment planning. Because CT and MRI permit the direct visualization of soft tissue structures, tumors can be precisely located, instead of approximated. These detailed images have been directly integrated with computer-based modulation of the radiation beam outline, a technique known as three-dimensional conformal radiation therapy. Contemporary imaging modalities, such as CT and MRI, have also been directly incorporated into radiation delivery machines, allowing for frequent confirmation of the tumor and patient positioning throughout the course of treatment. This approach, which may be applied to a number of radiation therapy techniques, is called image-guided radiation therapy (Figure 1). If critical healthy structures, such as nerves or vessels, are adjacent to or surrounded by the target tumor, the radiation beam may be subdivided into multiple component beams (“beamlets”), each of which may be modified individually; this technique is called intensity-modulated radiation therapy11–13 (Figure 2). Figure 3 illustrates a modern radiation treatment plan using intensity-modulated radiation therapy techniques.
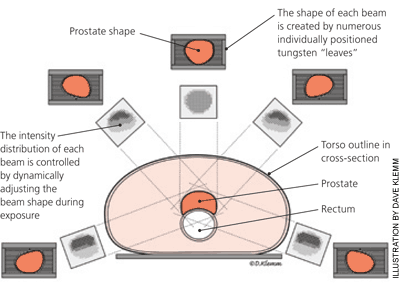
With three-dimensional conformal radiation therapy for prostate cancer, urinary and rectal toxicity rates have decreased substantially compared with conventional external beam radiation therapy14 ; these complications have declined even further with intensity-modulated radiation therapy.11,12 Tumors susceptible to repeated movement, such as those in the lungs, may be tracked and targeted with rapidly acquired anatomic images (four-dimensional radiation therapy).15,16
STEREOTACTIC RADIOTHERAPY AND RADIOSURGERY
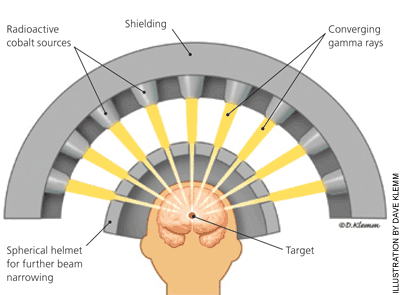
Despite substantial improvements in tumor targeting, technologies such as three-dimensional conformal and intensity-modulated radiation therapy are prone to the inherent uncertainties and limitations associated with dose fractionation. If the target tissue was immobile and its localization highly dependable, normal tissues would receive minimal radiation, thus decreasing or eliminating the need for fractionation. A single treatment is more convenient than six weeks of daily sessions. A one-time, high-potency radiation dose also provides greater tumor kill rates than an equal or higher radiation dose divided over multiple administrations because there is less opportunity for cancer cells to repair damage to DNA.
This is the concept behind stereotactic radiosurgery, a specialized type of external beam radiation therapy. Stereotactic radiosurgery (Figure 4) has primarily been used as an alternative to surgery for the treatment of intracranial lesions, such as brain metastases, arteriovenous malformations, acoustic neuromas, trigeminal neuralgia, and meningiomas.17–21 The brain is an ideal location for this approach because there is essentially no internal organ movement. The Gamma Knife stereotactic radiosurgery system involves attaching a positioning device (i.e., a stereotactic frame) directly to the patient, then to the treatment unit. Outside the brain, stereotactic body radiation therapy also relies on patient immobilization equipment for accurate targeting of tumors. The Cyberknife is a linear accelerator mounted on a robotic arm that provides more than 1,000 radiation beam orientations. It has been used to treat tumors in the lung, liver, spine, kidney, prostate, and pancreas.22,23 Although stereotactic radiation is often more effective than conventional radiation, the high radiation doses required for these treatments can lead to distinct radiation toxicities. Tubular structures, such as bronchi and bile ducts, are particularly prone to damage, which may manifest as luminal obliteration and obstruction.23
BRACHYTHERAPY
With brachytherapy, the radiation source is permanently or temporarily placed within the patient, near the target tumor. For example, permanent iodine-125 radiation seed implants have become an established treatment for early-stage, low-risk prostate cancer. Temporary brachytherapy, administered via intracavitary catheters or larger applicators, is used to treat gynecologic malignancies, such as cervical cancer. Balloon catheters, filled with liquid radioisotopes, are used to limit local recurrence after the initial treatment of breast cancer and brain tumors; they are placed during surgical resection, then removed after several days.24,25
SYSTEMIC RADIATION THERAPY
If adequate targeting is feasible, internal radiation may be administered systemically. Because thyroid tissue naturally concentrates iodine, iodine-131 may be given orally to treat localized and metastatic thyroid cancer26 or benign causes of hyperthyroidism. Although this treatment almost always leads to hypothyroidism requiring thyroid hormone replacement, other organs are not affected. Similarly, the radioisotopes strontium-89 and samarium-153, which have affinity for bone, have been used to palliate painful skeletal metastases from prostate, breast, and lung cancers.27 More recently, radioisotopes have been attached to monoclonal antibodies that target cancer cells (i.e., radioimmunotherapy) to treat non-Hodgkin lymphoma. Because these antibodies may be recognized as foreign proteins, patients must be closely monitored for infusion reactions.
Treatment Approach
CHOOSING RADIATION THERAPY
Radiation therapy may be presented to the patient as one of multiple treatment options. These options, which require multidisciplinary consultation and counseling, vary somewhat among diseases. For instance, localized prostate cancer is often treated with radical prostatectomy or radiation therapy. Rectal toxicities occur more commonly with radiation, whereas genitourinary complications occur more commonly with radical prostatectomy. Surgery lasts hours and provides immediate tumor removal, but has operative risks and discomforts. Radiation therapy lasts almost two months, and maximal prostate-specific antigen response does not occur until more than one year later28 ; however, no incision is required. Despite these differences, surgery and radiation therapy generally have similar outcomes and overall complications. Therefore, in the absence of medical comorbidities precluding surgery, the selection of treatment modality depends highly on patient preference.29–31 In contrast, for most patients with locally advanced laryngeal cancer, the choice of radiation versus surgery is more straightforward. Both treatment modalities have similar disease control rates1,2; however, because total laryngectomy leads to the inability to speak, most physicians and patients opt for radiation, when feasible.
The selection of a specific radiation technique is also highly individualized. The improvements in imaging and dose distribution that characterize three-dimensional conformal radiation therapy and intensity-modulated radiation therapy make these techniques particularly suitable for tumors in the vicinity of critical normal structures, such as with prostate cancer and head and neck cancer. Stereotactic body radiation therapy appears to be effective in the treatment of early-stage lung cancer in patients who are not medically fit to undergo surgery; however, excessive toxicities preclude its use for tumors located within 2 cm of the proximal bronchial tree.32 In addition to anatomic considerations, patient geography and economics also contribute to this decision. Although stereotactic and intensity-modulated radiation therapies are now available in most major metropolitan areas, they are typically not available in rural areas or smaller cities. For prostate cancer treatment, intensity-modulated radiation therapy may cost up to $50,000, compared with $10,000 to $25,000 for conventional external beam radiation therapy.33,34
RECEIVING RADIATION THERAPY
After an initial consultation with a radiation oncologist, the patient undergoes a treatment planning session (i.e., simulation). During this session, freckle-size tattoos or colored ink marks may be placed on the skin to guide the orientation of the radiation beam during treatment. A body mold or mesh face mask, to assist with patient immobilization, may be custom-fitted. Fractions are then delivered daily, Monday through Friday, for two to seven weeks depending on tumor type. Rarely, two smaller fractions may be administered daily (hyperfractionated radiation). For conventional external beam radiation therapy, each fraction takes only one to two minutes to deliver. For intensity-modulated radiation therapy, which requires frequent reorientation and reconfiguration of the radiation beam, it may take more than 30 minutes to deliver a fraction. Stereotactic radiation, which may be delivered as a single treatment or as three to five fractions administered every two to three days (for nonintracranial lesions), may take more than 45 minutes per fraction.
The radiation treatment itself is painless. However, discomfort may occur from attachment of a frame to the skull during stereotactic radiosurgery or from insertion of a catheter or applicator during brachytherapy. Patients undergoing external beam radiation therapy are not radioactive because the source of radiation remains outside the body; radiation exposure occurs only when the beam is turned on during the treatment session. In contrast, the radiation source is implanted within patients receiving brachytherapy. Temporary brachytherapy techniques may require hospitalization in a private room during the treatment course. Patients receiving radioimmunotherapy have circulating radioisotopes that are cleared from the bloodstream over several days. Although out-patient treatment is an option, patients receiving radioimmunotherapy must take radiation precautions (hand washing; careful disposal of body fluids, including urine, tears, and sweat; and condom use) for one week. With permanent brachytherapy devices, patients may be required to limit social contacts, particularly with children and pregnant women, for up to one month after implantation. Although the implants are never removed, the radiation dissipates within six months.
FOLLOW-UP
Because 75 percent of patients receiving radiation are treated with the intent to cure the cancer,35 family physicians are likely to care for these patients not only before and during their radiation treatment, but also in the years after treatment. Thus, an understanding of radiation toxicities (Table 336–39) will assist in the treatment of these patients.40 Localized skin changes and fatigue occur in many patients receiving external beam radiation therapy. Other adverse effects of radiation therapy depend largely on the anatomic site. Common toxicities include diarrhea, nausea and vomiting, mucositis, xerostomia, hair loss in the treatment area, and sexual and urinary changes. Plain, nonscented, lanolin-free hydrophilic cream can help patients who have radiation skin reactions.41 Most acute toxicities resolve within the two months after the completion of radiation therapy, although some persist indefinitely.
| Healthy tissue at risk | Toxicities* | Monitoring/prevention | Treatment |
|---|---|---|---|
| Brain | |||
| Optic nerves, chiasm, lens, retina | Late: blindness, optic neuritis, cataracts, retinal atrophy | — | Cataract removal |
| Brainstem | Acute: edema Late: motor and sensory dysfunction, stroke, radionecrosis | Follow-up MRIs | Corticosteroids |
| Brain tissue | Acute: edema, fatigue, nausea | Follow-up MRIs | Corticosteroids |
| Late: radionecrosis, memory loss | Surgery for persistent, symptomatic necrosis | ||
| Hair | Acute/late: alopecia | — | Scalp protection when outdoors |
| Head and neck | |||
| Salivary glands | Acute/late: xerostomia | Amifostine (Ethyol; an intravenous cytoprotective agent),38 smoking cessation, gargling often with salt or baking soda solution | Saliva substitutes; saliva stimulants (sialogogues, such as pilocarpine [Salagen]) |
| Mucous membranes | Acute: mucositis Late: dysphagia | Smoking cessation | Topical anesthetics (viscous lidocaine [Xylocaine]), systemic analgesics |
| Teeth and gums | Acute: infection, decay Late: osteoradionecrosis | Dental evaluation and treatment (e.g., extraction of diseased teeth) before starting radiation and throughout follow-up39 | Hyperbaric oxygen |
| Pharyngeal muscles | Late: swallowing dysfunction, speech problems | Jaw muscle exercises | Speech and swallowing therapy |
| Thyroid | Late: hypothyroidism | Thyroid-stimulating hormone tests every six to 12 months after completion of radiation therapy | Thyroid hormone supplementation |
| Thorax | |||
| Lungs | Acute: pneumonitis Late: loss of lung capacity, fibrosis | Smoking cessation | Acute: corticosteroids; chronic: supplemental oxygen, pentoxifylline (Trental), vitamin E |
| Heart | Late: pericarditis, coronary artery disease | — | Coronary stents, cardiac surgery |
| Esophagus | Acute: esophagitis Late: esophagus stricture | — | Topical or systemic analgesics Esophagus dilatation |
| Spinal cord | Late: paralysis, myelitis | — | Corticosteroids |
| Abdomen | |||
| Liver | Late: liver damage | — | — |
| Stomach | Acute: nausea, vomiting Late: ulceration | Antiemetic agents Proton pump inhibitors | Antiemetic agents Proton pump inhibitors |
| Small bowel | Acute: nausea, vomiting, diarrhea Late: small bowel obstruction | Antiemetic agents | Antiemetic agents, antidiarrheal agents (e.g., loperamide [Imodium]) |
| Kidney | Late: renal failure | — | — |
| Pelvis | |||
| Rectum | Acute: proctitis, rectal bleeding Late: rectal bleeding, rectal stricture | Sitz baths | Hydrocortisone cream Rectal dilatation |
| Bladder | Acute: cystitis, incontinence, urinary frequency, bladder spasms Late: urethral stricture, bladder contracture | — | Alpha blockers (e.g., tamsulosin [Flomax]), urethral dilatation for strictures |
| Vagina | Acute: vaginal dryness and irritation Late: vaginal stenosis or stricture | Lubrication during sexual intercourse | Vaginal dilatation |
| Ovaries, testes | Late: infertility | Sperm or egg preservation | — |
| Prostate | Acute: urinary obstruction | — | Alpha blockers |
| Sexual function (men) | Late: impotence | — | Phosphodiesterase inhibitors (e.g., sildenafil [Viagra]), penile implant |
| Bone marrow | Acute/late: myelosuppression | Weekly blood counts | Erythropoietin therapy Myeloid growth factors (e.g., granulocyte-colony-stimulating factor) |
| Extremities | |||
| Joints | Late: scar tissue accumulation, loss of motion | — | Physical therapy, surgery |
| Lymph nodes | Late: lymphedema | — | Extremity wraps, physical therapy |
Late toxicities may appear years after the completion of radiation therapy and, as with acute toxicities, vary by radiation field and dose. Late effects may include lymphedema, joint problems, xerostomia, infertility, cognitive changes, and, rarely, secondary malignancies. Men and women wishing to have children after receiving pelvic radiation may consider sperm or egg preservation before starting treatment. Because radiation is teratogenic in all stages of pregnancy, birth control is essential for women who could become pregnant.5,42 Follow-up after radiation therapy, which consists of regular physician visits, radiologic studies, or serum tumor markers, focuses on toxicity management and detecting cancer recurrence.