
A more recent article on anemia in infants and children is available.
Am Fam Physician. 2010;81(12):1462-1471
Author disclosure: Nothing to disclose.
Anemia is defined as a hemoglobin level of less than the 5th percentile for age. Causes vary by age. Most children with anemia are asymptomatic, and the condition is detected on screening laboratory evaluation. Screening is recommended only for high-risk children. Anemia is classified as microcytic, normocytic, or macrocytic, based on the mean corpuscular volume. Mild microcytic anemia may be treated presumptively with oral iron therapy in children six to 36 months of age who have risk factors for iron deficiency anemia. If the anemia is severe or is unresponsive to iron therapy, the patient should be evaluated for gastrointestinal blood loss. Other tests used in the evaluation of microcytic anemia include serum iron studies, lead levels, and hemoglobin electrophoresis. Normocytic anemia may be caused by chronic disease, hemolysis, or bone marrow disorders. Workup of normocytic anemia is based on bone marrow function as determined by the reticulocyte count. If the reticulocyte count is elevated, the patient should be evaluated for blood loss or hemolysis. A low reticulocyte count suggests aplasia or a bone marrow disorder. Common tests used in the evaluation of macrocytic anemias include vitamin B12 and folate levels, and thyroid function testing. A peripheral smear can provide additional information in patients with anemia of any morphology.
An estimated 20 percent of American children will have anemia at some point in their childhood.1 Anemia is defined as a hemoglobin (Hgb) concentration or red blood cell (RBC) mass less than the 5th percentile for age. Hgb levels vary by age, and many laboratories use adult norms as references; therefore, the patient's Hgb level must be compared with age-based norms to diagnose anemia2 (Table 13).
Anemia is usually classified based on the size of RBCs, as measured by the mean corpuscular volume (MCV). Anemia can be microcytic (MCV typically less than 80 μm3 [80 fL]), normocytic (80 to 100 μm3 [80 to 100 fL]), or macrocytic (greater than 100 μm3 [100 fL]). The RBC distribution width is a measure of the size variance of RBCs. A low RBC distribution width suggests uniform cell size, whereas an elevated width (greater than 14 percent) indicates RBCs of multiple sizes.
| Clinical recommendation | Evidence rating | References |
|---|---|---|
| Screening for anemia in high-risk infants and toddlers is recommended; universal screening is not. | B | 9, 18, 19 |
| If anemia is consistent with iron deficiency in a child six to 36 months of age with low mean corpuscular volume and elevated red blood cell distribution width, it is reasonable to try oral iron therapy for one month before additional diagnostic testing. | C | 9, 18, 23 |
| Iron deficiency anemia should be treated with oral iron therapy. | C | 9, 18 |
| Iron deficiency (with or without anemia) is associated with negative behavioral and cognitive effects that may not be reversible. | C | 28–33 |
| To prevent iron deficiency anemia, adequate dietary iron intake should be ensured in infants older than six months, and cow's milk should be limited to 16 to 24 oz per day in children older than 12 months. | C | 9, 22 |
| Age | Hemoglobin (g per dL) | Hematocrit (%) | Mean corpuscular volume (fL) | |||
|---|---|---|---|---|---|---|
| Mean | 2 SDs below mean | Mean | 2 SDs below mean | Mean | 2 SDs below mean | |
| 26 to 30 weeks' gestation | 13.4 | 11.0 | 41.5 | 34.9 | 118.2 | 106.7 |
| 28 weeks' gestation | 14.5 | NA | 45 | NA | 120 | NA |
| 32 weeks' gestation | 15.0 | NA | 47 | NA | 118 | NA |
| Full term (cord sample) | 16.5 | 13.5 | 51 | 42 | 108 | 98 |
| 1 to 3 days | 18.5 | 14.5 | 56 | 45 | 108 | 95 |
| 2 weeks | 16.6 | 13.4 | 53 | 41 | 105 | 88 |
| 1 month | 13.9 | 10.7 | 44 | 33 | 101 | 91 |
| 2 months | 11.2 | 9.4 | 35 | 28 | 95 | 84 |
| 6 months | 12.6 | 11.1 | 36 | 31 | 76 | 68 |
| 6 months to 2 years | 12.0 | 10.5 | 36 | 33 | 78 | 70 |
| 2 to 6 years | 12.5 | 11.5 | 37 | 34 | 81 | 75 |
| 6 to 12 years | 13.5 | 11.5 | 40 | 35 | 86 | 77 |
| 12 to 18 years (male) | 14.5 | 13.0 | 43 | 36 | 88 | 78 |
| 12 to 18 years (female) | 14.0 | 12.0 | 41 | 37 | 90 | 78 |
| Adult (male) | 15.5 | 13.5 | 47 | 41 | 90 | 80 |
| Adult (female) | 14.0 | 12.0 | 41 | 36 | 90 | 80 |
Etiology
Although some studies have suggested a decline in the prevalence of anemia,4,5 the most recent Pediatric Nutrition Surveillance System Report showed an increase among low-income children, from 13 percent in 2002 to 15 percent in 2007.6 The causes of anemia vary by age (Table 2).2,7 Anemia should not be considered a diagnosis, but a finding that warrants further investigation.8 In children, it is usually caused by decreased RBC production or increased RBC turnover.2
| Cause | Etiology and epidemiology | Presentation | Indices and other laboratory testing |
|---|---|---|---|
| Neonatal7 | |||
| Blood loss | Hemorrhage (placental abruption, subgaleal, traumatic); maternal-fetal and twin-twin transfusion | Tachypnea, pallor, and mental status change (irritability, poor feeding); > 20 percent loss of blood volume results in shock and cardiopulmonary collapse | Anemia with normal indices; reticulocyte count is initially normal, then increases; positive Kleihauer-Betke test in maternal-fetal hemorrhage |
| Accounts for 5 to 10 percent of all cases of severe neonatal anemia | |||
| Isoimmunization | ABO incompatibility, Rh incompatibility | Jaundice and mild anemia; infants with severe isoimmunization (e.g., untreated Rh incompatibility) may present with hydrops fetalis | Positive Coombs test; elevated bilirubin level; normocytic anemia with elevated reticulocyte count |
| Rh incompatibility occurs in 10.6 per 10,000 live births; 50 percent of these infants develop anemia | |||
| Congenital hemolytic anemia | Spherocytosis, G6PD deficiency | Hyperbilirubinemia and moderate jaundice | Low enzyme activity; with hemolysis, smear may show poikilocytosis, reticulocytosis, Heinz bodies, and bite cells (in G6PD deficiency) or spur cells (in pyruvate kinase deficiency) |
| Congenital infection | Parvovirus B19, human immunodeficiency virus, syphilis, rubella, sepsis | Pallor, irritability, and other findings associated with infection (e.g., deafness) | Normocytic anemia with low reticulocyte count |
| Diamond-Blackfan syndrome | Congenital pure red cell aplasia resulting from increased apoptosis in erythroid precursors | Neonatal pallor progressing to symptomatic anemia; average age of diagnosis is 3 months; about 30 percent have other abnormalities | Macrocytic anemia with low reticulocyte count |
| Affects 7 per 1 million live births | |||
| Fanconi anemia | Increased susceptibility of progenitor cells in bone marrow leads to increased apoptosis, progressing to pancytopenia | Average age of diagnosis is 8 years, but associated congenital abnormalities may facilitate early diagnosis (e.g., café-au-lait spots; microsomy; low birth weight; thumb, renal, skeletal, and eye abnormalities) | Microcytic anemia and reticulocytopenia, thrombocytopenia, or leukopenia; DNA sequencing can detect genetic mutations for Fanconi anemia complementation groups |
| Infancy to toddlerhood2 | |||
| Iron deficiency | Inadequate dietary intake, chronic occult blood loss (excessive cow's milk consumption, inflammatory bowel disease, Meckel diverticulum, parasites) | Usually asymptomatic; severe cases can present with fatigue, pallor, or dyspnea; rarely occurs before 6 months of age; highest risk is at 6 to 36 months of age | Microcytic anemia with elevated RBC distribution width; peripheral smear shows hypochromic microcytes and may show target cells; iron and ferritin levels and iron saturation are low; transferrin level is elevated |
| Prevalence is 8 to 15 percent | |||
| Concurrent infection | Bacterial or viral infection leading to cytokine-mediated decrease in iron utilization and RBC production | Presenting symptoms usually result from infectious process | Normocytic or mildly microcytic, low/normal serum iron level with low transferrin level; ferritin level may be elevated because it is an acute phase reactant |
| Blood loss | Trauma, gastrointestinal bleeding | Tachypnea, tachycardia, pallor, hypotension | Hgb levels may initially be normal, followed by anemia with normal indices |
| Disorder of Hgb structure or synthesis | Thalassemia, sickle cell disease | Anemia in thalassemia may range from mild and asymptomatic to severe, depending on number of heme chains affected; sickle cell disease presents with hemolysis, pain crises, dactylitis, and aplastic crisis; symptoms are rarely present at birth but typically develop in the first year | Microcytic anemia, low RBC distribution width, and low Mentzer index in thalassemia; Hgb electrophoresis may show Hgb F; smear with basophilic stippling; hemolysis, reticulocytosis, and Hgb S on electrophoresis in sickle cell disease |
| RBC enzyme defects | G6PD deficiency, pyruvate kinase deficiency | Neonatal hyperbilirubinemia and hemolytic anemia when exposed to oxidative stress | Low enzyme activity; with hemolysis smear may show poikilocytosis, reticulocytosis, Heinz bodies, and bite cells (in G6PD deficiency) or spur cells (in pyruvate kinase deficiency) |
| 10 percent of the black population has G6PD deficiency | |||
| RBC membrane defects | Spherocytosis, elliptocytosis | Hyperbilirubinemia, splenomegaly, gall bladder disease, and aplastic crisis; autosomal dominant, so family history is positive in about 75 percent of patients | Macrocytosis, reticulocytosis, elevated bilirubin and lactate dehydrogenase levels; spherocytes or elliptocytes on smear; osmotic fragility test is commonly done but not specific |
| Acquired hemolytic anemias | Antibody-mediated hemolysis, drug-induced hemolysis, hemolytic uremic syndrome, disseminated intravascular coagulation | Jaundice, fatigue, dyspnea | Positive Coombs test and spherocytes visible on smear in antibody-mediated hemolysis; schistocytes visible on smear in hemolytic uremic syndrome or disseminated intravascular coagulation |
| Transient erythro-blastopenia of childhood | Transient immune reaction against erythroid progenitor cells | Anemia after toxin ingestion or viral illness, usually in children 6 months to 3 years of age | Normocytic anemia, initially with reticulocyte count of 0; anemia resolves within 2 months |
| Leukemia, myelofibrosis | Usually spontaneous, but rates are increased in patients with prior radiation exposure or chemotherapy | Anemia causes pallor, fatigue, and dyspnea; patients with leukemia may present with petechiae, low-grade fever, nonspecific bone pain, gum swelling, or rash | Normocytic anemia with decreased reticulocyte count; leukopenia, leukocytosis, or thrombocytopenia; peripheral smear shows blast cells |
| Lead poisoning | Risk factors include young age, living in a home built before 1970 or in areas where soil is contaminated, and pica (as in iron deficiency) | In addition to anemia, patients may present with abdominal pain, altered mental status, renal disease, and hypertension | Microcytic anemia may be concurrent with iron deficiency; peripheral smear may show basophilic stippling; hemolysis may be present |
| Late childhood and adolescence2 | |||
| Iron deficiency | Second peak in iron deficiency occurs in adolescence because of growth spurt, menstruation, and poor dietary iron intake | Pallor, fatigue, dyspnea | Same as for infants and toddlers, above |
| Chronic disease | Renal disease, liver disease, hypothyroidism, other chronic illnesses | Usually mild and asymptomatic | Normocytic or mildly microcytic, low/normal serum iron level with low transferrin level; ferritin level may be elevated because it is an acute phase reactant |
| Blood loss | Same as for infants and toddlers, above | ||
| Menstruation in adolescent girls | |||
| Disorders of Hgb synthesis or RBC membrane defects | Same as for infants and toddlers, above | ||
| Acquired hemolytic anemias | Same as for infants and toddlers, above | ||
| Leukemia and other bone marrow disorders | Same as for infants and toddlers, above | ||
Iron deficiency commonly causes decreased RBC production. Risk factors include prematurity, poor diet, consumption of more than 24 oz of cow's milk per day, and chronic blood loss.9 Other causes of decreased RBC production include inflammation from chronic infection or other inflammatory conditions, renal failure, medication use, viral illnesses, and bone marrow disorders (Table 3).2,10
Increased RBC turnover may be a result of blood loss, mechanical destruction of RBCs, or hemolysis. Hemolysis may result from inherited defects in RBCs; therefore, sex, ethnicity, and family history are potential risk factors. Medications may cause anemia because of immune-mediated hemolysis or oxidative stress. Mechanical destruction may occur in persons with mechanical valves or splenomegaly. RBC loss may also be a result of acute bleeding.2
| Etiology | Risk factor | Comment |
|---|---|---|
| Decreased RBC production | Chronic disease | Renal disease can result in anemia because of decreased erythropoietin levels; hypothyroidism can result in macrocytic anemia because of impaired RBC production; chronic inflammation (as in chronic infection or rheumatologic disease) can lead to cytokine-mediated suppression of erythropoiesis; inflammatory bowel disease or celiac disease can result in anemia because of inflammation and nutrient malabsorption |
| Iron deficiency10 | Pica induced by iron deficiency increases risk of lead ingestion, and lead is absorbed more readily in the presence of iron deficiency; iron levels should be tested in patients with lead poisoning | |
| Poor diet | Inadequate nutrient intake can cause deficiencies in iron, folate, and vitamins A, B12, and D | |
| Prematurity | Decreased iron stores and increased demand for catch-up growth can cause iron deficiency; rarely occurs before birth weight is doubled | |
| Increased RBC turnover | Drug use | Primaquine, sulfamethoxazole, and nitrofurantoin (Furadantin) can lead to hemolysis; this is more pronounced in patients with G6PD deficiency but can occur in any patient; phenytoin (Dilantin) can cause megaloblastic anemia |
| Ethnicity | African ancestry in sickle cell disease; Mediterranean, Asian, or African ancestry in thalassemia; Sephardic Jewish, Filipino, Greek, Sardinian, or Kurdish ancestry in G6PD deficiency | |
| Family history | Thalassemia, spherocytosis, and sickle cell disease; family history may include gallstones and jaundice in addition to anemia | |
| Mechanical heart valves | Mechanical destruction by the valve can cause hemolysis | |
| Sex | G6PD deficiency and pyruvate kinase deficiency are X-linked and therefore more common in males | |
| Splenomegaly | Sequestration and increased destruction of RBCs can cause hemolysis | |
| Both | Infection | Infection can precipitate immune-mediated hemolytic anemia or cause hemolytic crises in patients with inherited enzyme defects and sickle cell disease; can cause RBC aplasia (as in parvovirus B19 infection) or result in transient erythroblastopenia of childhood |
Diagnosis
CLINICAL DIAGNOSIS
Most children with mild anemia have no signs or symptoms. Some may present with irritability or pica (in iron deficiency), jaundice (in hemolysis), shortness of breath, or palpitations. Physical examination may show jaundice, tachypnea, tachycardia, and heart failure, especially in children with severe or acute anemia.
Pallor has poor sensitivity for predicting mild anemia, but correlates well with severe anemia.11–13 One study showed that physical examination findings of pallor of the conjunctivae, tongue, palm, or nail beds is 93 percent sensitive and 57 percent specific for the diagnosis of anemia in patients with an Hgb level of less than 5 g per dL (50 g per L).14 The sensitivity decreases to 66 percent when the Hgb level is 5 to 8 g per dL (50 to 80 g per L).14 Chronic anemia may be associated with glossitis, a flow murmur, and growth delay, although these conditions are rare in developed countries.1
DIAGNOSTIC TESTS
Laboratory tests used in the diagnosis of anemia include measurement of ferritin, which reflects iron stores, and transferrin or total iron-binding capacity, which indicates the body's ability to transport iron for use in RBC production.
Hgb measurement fails to detect many cases of early or mild iron deficiency because the life span of RBCs reflect bone marrow iron content from up to 120 days previously. Because reticulocytes survive in the periphery for only one or two days, reticulocyte hemoglobin content (RHC) is a more accurate “real-time” measurement of bone marrow iron status.15 Alternatively, many cases of anemia in children are not caused by iron deficiency. Therefore, measurement of a single Hgb level may result in unnecessary treatment and retesting.16 Measurement of RHC may help avoid this issue. In a study of infants nine to 12 months of age, an Hgb level of less than 11 g per dL (110 g per L) was only 26 percent sensitive in detecting iron deficiency (as measured by a transferrin saturation of less than 10 percent), whereas an RHC of less than 27.5 pg was 83 percent sensitive in detecting iron deficiency.17 RHC is not available in all laboratories, and more studies are needed to determine whether screening with this test is clinically useful and cost-effective.
Approach to the Child with Anemia: Illustrated Case Studies
ANEMIA IN A NEWBORN
A full-term infant is delivered with the use of forceps; the pregnancy and delivery were otherwise uncomplicated. The initial examination is normal, but on the second hospital day, he is pale and fussy. The reticulocyte count and bilirubin level are normal, and the Hgb level is 9 g per dL (90 g per L). Repeat physical examination reveals an increased head circumference.
Causes of anemia in the newborn are blood loss, decreased RBC production, and increased RBC turnover. Blood loss during delivery can result from a ruptured umbilical cord, placenta previa, and abruptio placentae. Maternal-fetal transfusion occurs in 50 percent of all pregnancies, but usually does not cause significant loss of blood volume.7 The patient's history eliminates most of these causes.
A normal reticulocyte count confirms that the infant's bone marrow is functional. This rules out causes of decreased RBC production, including Fanconi anemia, Diamond-Blackfan syndrome, and congenital infections.
Cranial hemorrhages are often associated with birth trauma, including vacuum and forceps delivery. In particular, subgaleal bleeds can be of sufficient volume to cause shock. Physical examination findings may include mental status changes, jaundice, tachycardia or tachypnea, and increased head circumference.7
In this patient, a computed tomography scan confirms a subgaleal hemorrhage, and the infant is transferred to a neonatal intensive care unit for transfusion and monitoring.
In newborns, an elevated bilirubin level in association with anemia suggests hemolysis. If this infant's biliru-bin level had been elevated, further testing would have included a Coombs test to evaluate for isoimmunization (as in ABO or Rh incompatibility) and a peripheral smear to evaluate for spherocytosis or other RBC membrane defects. Testing for glucose-6-phosphate dehydrogenase (G6PD) deficiency should be considered if the patient's ethnicity or family history is a risk factor.
MICROCYTIC ANEMIA IN AN INFANT
A 12-month-old boy of Mediterranean descent presents for a health maintenance examination. He consumes 32 oz of whole milk daily. The medical history and review of systems are normal. On physical examination, the patient is found to have an elevated weight for length. No other abnormalities are noted. Laboratory testing shows that the patient's Hgb level is 9.8 g per dL (98 g per L). The MCV is low (70 μm3 [70 fL]), and the RBC distribution width is elevated (18 percent). The RBC count is 5.0×106 per mm3 (5.0×1012 per L). The child is presumptively treated with oral iron therapy, and after one month, the Hgb level is 11.2 g per dL (112 g per L). After another month of iron therapy, the Hbg level has normalized at 13 g per dL (130 g per L).
Neither the Centers for Disease Control and Prevention, the American Academy of Pediatrics, nor the U.S. Preventive Services Task Force recommends universal screening for anemia. Instead, children at risk should be identified and then undergo evaluation between nine and 12 months of age (Table 4).9,18,19 This child's excessive milk consumption and weight are risk factors for anemia20–22; therefore, evaluation is justified.
| Organization | Recommendations | High-risk groups |
|---|---|---|
| American Academy of Pediatrics | Screening is recommended at 9 to 12 months of age and again 6 months later for all infants in populations with high rates of iron deficiency, or (in populations with a rate of 5 percent or less) in infants with medical risks or whose diet puts them at risk of iron deficiency |
|
| Centers for Disease Control and Prevention | Screening is recommended for children from low-income or newly immigrated families between 9 and 12 months of age, then 6 months later, then annually from 2 to 5 years of age |
|
| Screening should be considered for preterm and low–birth-weight infants before 6 months of age if they are not fed iron-fortified formula | ||
| Infants and young children with risk factors should be assessed at 9 to 12 months of age, and again 6 months later | ||
| Beginning in adolescence, all nonpregnant women should be screened every 5 to 10 years | ||
| U.S. Preventive Services Task Force | No recommendation for or against screening for iron deficiency anemia in asymptomatic children 6 to 12 months of age |
|
| Screening at 9 to 12 months of age is recommended for high-risk infants |
Iron deficiency is characterized by microcytosis with an elevated RBC distribution width. Because the anemia is mild and the history and laboratory values are consistent with iron deficiency, it is appropriate to treat presumptively with oral iron therapy and repeat testing in one month23 (Figure 1). Treatment for mild anemia is 3 to 6 mg of elemental iron per kg per day.24 Once-daily dosing results in similar improvement as two- or three-times-daily dosing and does not significantly increase adverse effects.25
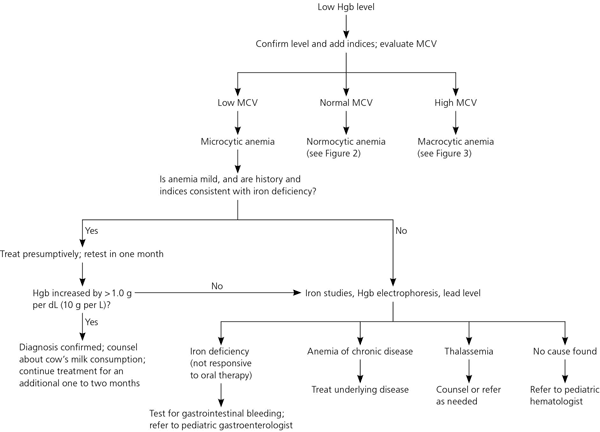
An Hgb increase of more than 1 g per dL (10 g per L) after iron therapy has been started confirms the diagnosis of iron deficiency. If the Hgb level does not increase or if the initial anemia is severe, further evaluation should include a complete blood count (CBC), peripheral blood smear, iron studies, and fecal occult blood testing. Lead testing should also be considered.
| Example patient | MCV(fL) | RBC count (×106 per mm3 ) | Mentzer index (MCV/RBC count) | Comments |
|---|---|---|---|---|
| 5-year-old black child with pallor | 64 | 5.3 | 12 | Mentzer index < 13 suggests thalassemia |
| 2-year-old child who drinks 30 oz of cow's milk daily | 72 | 4.8 | 15 | Mentzer index > 13 suggests iron deficiency |
Sideroblastic anemia, which is rare, results in a high RBC distribution width with normal or elevated iron levels; diagnosis requires bone marrow aspiration. Iron is utilized by tissues other than bone marrow, including the brain. Studies show an association between iron deficiency and impaired neurocognitive performance.28–33 The association is not definitively causal, and studies do not show an immediate improvement in psychomotor development or cognitive performance after treatment has commenced. However, long-term studies are few and conflicting.34 Until further studies provide clarity, iron deficiency should be treated until one month after normalization of Hgb levels. The total treatment course is typically three months. If a longer course is needed, further investigation should include a CBC, peripheral blood smear, iron studies, and fecal occult blood testing.23
NORMOCYTIC ANEMIA IN AN OLDER CHILD
A previously healthy eight-year-old boy of Filipino descent presents with increasing fatigue for the past five days. He has low-grade fever and nonspecific musculoskeletal pain. He has had no symptoms of upper respiratory infection. Physical examination shows pallor, pale conjunctivae, scattered facial petechiae, tachycardia, and a flow murmur. There is no scleral icterus. A CBC shows an Hgb level of 7.8 g per dL (78 g per L) and an MCV of 90 μm3 (90 fL). The white blood cell count is 14,000 per mm3 (14.00×109 per L), and the platelet count is 368×103 per mm3 (368×109 per L). The reticulocyte count is 0.21 percent (normal range in an eight-year-old is 0.5 to 1.0 percent). The peripheral smear shows 21 percent lymphoblasts.
This is normocytic anemia in a previously healthy child. Although normocytic anemia commonly results from early iron deficiency or chronic disease, this patient has findings suggesting an acute process (pallor, tachycardia, and flow murmur). Hemoglobinopathies, enzyme defects, RBC membrane defects, and other hemolytic anemias result in normocytic anemia. Given his sex and ethnicity, G6PD deficiency is in the differential diagnosis. However, he has no history and is not jaundiced, which makes hemolysis unlikely.
In a child who otherwise appears well and has had a recent viral infection, transient erythroblastopenia of childhood (TEC) should be considered. This condition usually occurs in children six months to three years of age after a viral infection or exposure to toxic agents. It is the result of an immune reaction against erythroid progenitor cells. In patients with TEC, the initial reticulocyte count is zero, but slowly increases as the patient recovers, which typically occurs within two months of onset.35 This child's age, ill appearance, and lack of viral symptoms make TEC less likely.
The first step in evaluation of normocytic anemia is determination of the reticulocyte count (Figure 2) to distinguish cases of increased RBC turnover, such as hemolysis, from bone marrow disorders. The low reticulocyte count suggests bone marrow hypofunction. Leukemia and aplastic anemia reduce RBC production. Because leukemia is a consideration in the differential diagnosis for this patient, a peripheral smear is ordered, which confirms the diagnosis of leukemia.
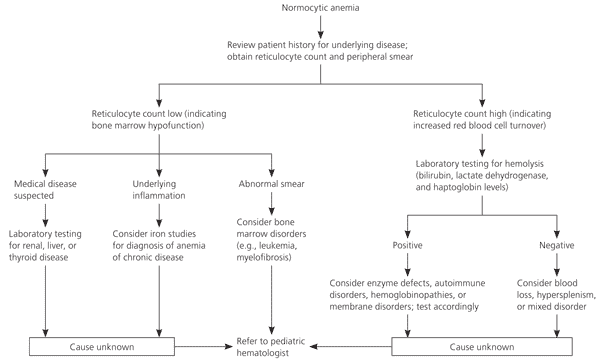
If the diagnosis had been less clear, further evaluation would have included a careful history and testing of iron levels and liver, kidney, and thyroid function to assess for chronic disease. Low iron saturation suggests early iron deficiency. Normal or elevated iron saturation in the presence of low serum iron levels suggests infection or chronic disease.
Other Considerations
Macrocytic anemia is rare in children. The initial workup is a peripheral smear (Figure 3).36 The presence of hyper-segmented neutrophils signals a megaloblastic anemia, which is caused by folate or vitamin B12 deficiency or other disorders of DNA synthesis. Nonmegaloblastic causes of macrocytosis include alcoholism, hemolysis, hemorrhage, hepatic disease, bone marrow disorders (e.g., aplastic anemia, myelodysplasia, sideroblastic anemia), and hypothyroidism. Subsequent testing is based on peripheral smear findings.36
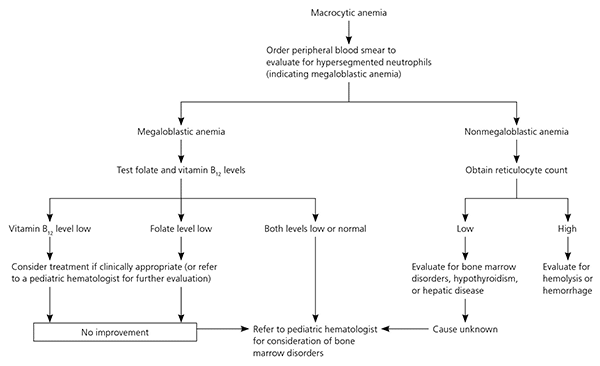
Older children and adolescents are also at risk of anemia. The combination of a growth spurt and the onset of menstruation leaves adolescent girls at particularly high risk of iron deficiency anemia.
Treatment and Prevention
Iron deficiency is treated orally; otherwise, treatment is geared toward the underlying cause of anemia. Symptomatic patients and those with severe anemia should receive a blood transfusion while evaluation for the underlying cause is undertaken. Transfusion is typically given at a volume of 10 mL per kg, infused at a rate of no more than 5 mL per kg per hour. The patient should be monitored for signs of heart failure during transfusion.
The U.S. Food and Drug Administration recommends adequate iron intake to prevent iron deficiency anemia (Table 69). One half of American toddlers do not receive the recommended daily intake of iron.37 However, it is not clear whether iron supplementation reduces the incidence of anemia. Studies in countries outside the United States have had promising results. However, a randomized study in the United States demonstrated that high-risk, six-month-old infants who received 10 mg of supplemental iron per day did not have a reduced incidence of anemia or abnormal indices indicative of iron deficiency.38
| Age | Daily iron requirement | Source |
|---|---|---|
| Up to 4 to 6 months (full-term infants) | 0.27 mg | Breast milk or iron-fortified formula |
| 4 to 6 months to 1 year (full-term infants) | 11 mg | Breast milk or formula plus iron-rich foods* |
| 1 month to 1 year (premature or low–birth-weight infants) | 2 to 4 mg per kg | Iron-fortified preterm formula or iron supplementation (2 mg per kg per day) plus breast milk and iron-rich foods |
| 1 to 3 years | 7 mg | Iron-rich foods |
In the first four to six months of life, full-term infants use hepatic stores of iron in addition to dietary iron in formula or breast milk; iron supplementation is not required in these children. Preterm infants do not have adequate hepatic iron stores and require larger amounts of iron for catch-up growth. These infants should receive supplemental iron. Starting at four to six months of age, infants require an additional source of iron.39 One half cup of iron-fortified cereal contains 90 percent of the recommended daily intake of iron for a six-to 12-month-old infant. Lean meats, beans, iron-fortified whole grains, tofu, and spinach are other iron-rich options for infants who consume solid foods.
