
Am Fam Physician. 2011;83(10):1197-1200
Summary of Recommendations and Evidence
The U.S. Preventive Services Task Force (USPSTF) recommends screening for osteoporosis in women 65 years or older and in younger women whose fracture risk is equal to or greater than that of a 65-year-old white woman with no additional risk factors (Table 1). B recommendation.
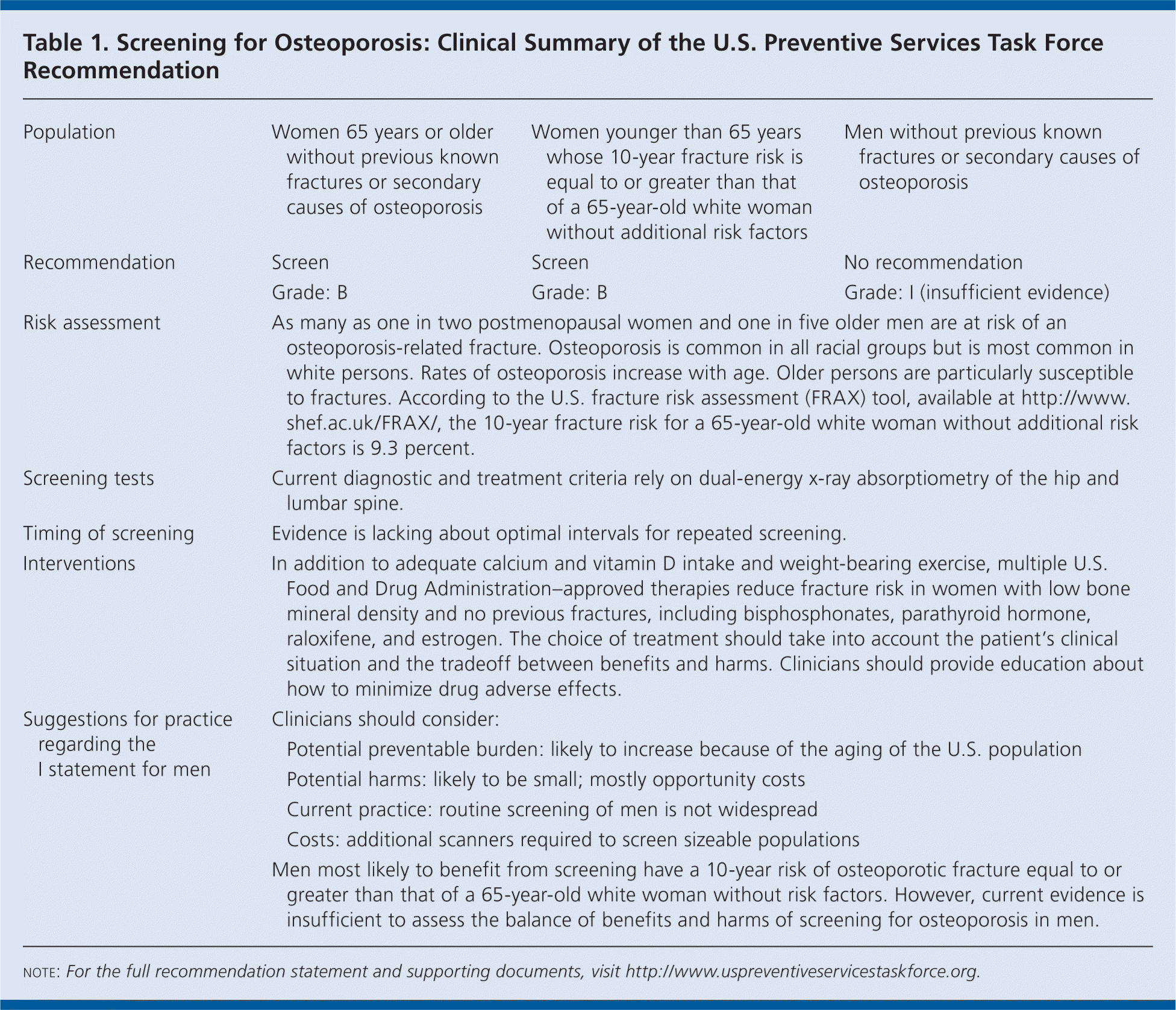
| Population | Women 65 years or older without previous known fractures or secondary causes of osteoporosis | Women younger than 65 years whose 10-year fracture risk is equal to or greater than that of a 65-year-old white woman without additional risk factors | Men without previous known osteoporosis | |
| Recommendation | Screen | Screen | No recommendation | |
| Grade: B | Grade: B | Grade: I (insufficient evidence) | ||
| Risk assessment | As many as one in two postmenopausal women and one in five older men are at risk of an osteoporosis-related fracture. Osteoporosis is common in all racial groups but is most common in white persons. Rates of osteoporosis increase with age. Older persons are particularly susceptible to fractures. According to the U.S. fracture risk assessment (FRAX) tool, available at http://www.shef.ac.uk/FRAX/, the 10-year fracture risk for a 65-year-old white woman without additional risk factors is 9.3 percent. | |||
| Screening tests | Current diagnostic and treatment criteria rely on dual-energy x-ray absorptiometry of the hip and lumbar spine. | |||
| Timing of screening | Evidence is lacking about optimal intervals for repeated screening. | |||
| Interventions | In addition to adequate calcium and vitamin D intake and weight-bearing exercise, multiple U.S. Food and Drug Administration–approved therapies reduce fracture risk in women with low bone mineral density and no previous fractures, including bisphosphonates, parathyroid hormone, raloxifene, and estrogen. The choice of treatment should take into account the patient's clinical situation and the tradeoff between benefits and harms. Clinicians should provide education about how to minimize drug adverse effects. | |||
| Suggestions for practice regarding the I statement for men | Clinicians should consider:
| |||
| Men most likely to benefit from screening have a 10-year risk of osteoporotic fracture equal to or greater than that of a 65-year-old white woman without risk factors. However, current evidence is insufficient to assess the balance of benefits and harms of screening for osteoporosis in men. | ||||
The USPSTF concludes that the current evidence is insufficient to assess the balance of benefits and harms of screening for osteoporosis in men. I statement.
Rationale
Importance. By 2012, approximately 12 million Americans older than 50 years are expected to have osteoporosis. One-half of all post-menopausal women will have an osteoporosis-related fracture during their lifetime; 25 percent of these women will develop a vertebral deformity, and 15 percent will experience a hip fracture. Osteoporotic fractures, particularly hip fractures, are associated with chronic pain and disability, loss of independence, decreased quality of life, and increased mortality. Although hip fractures are less common in men than in women, more than one-third of men who experience a hip fracture die within one year.
Detection. The USPSTF found convincing evidence that bone measurement tests predict short-term risk of osteoporotic fractures in women and men. The most commonly used tests are dual-energy x-ray absorptiometry (DXA) of the hip and lumbar spine, and quantitative ultrasonography of the calcaneus. Adequate evidence indicates that clinical risk assessment instruments have only modest predictive value for low bone density or fractures.
Benefits of detection and early intervention. No controlled studies have evaluated the effect of screening for osteoporosis on fracture rates or fracture-related morbidity or mortality.
In postmenopausal women who have no previous osteoporotic fractures, the USPSTF found convincing evidence that drug therapies reduce the risk of fractures. In women 65 years or older and in younger women whose fracture risk is equal to or greater than that of a 65-year-old white woman with no additional risk factors, the USPSTF judged that the benefit of treating screening-detected osteoporosis is at least moderate.
Because of the lack of relevant studies, the USPSTF found inadequate evidence that drug therapies reduce the risk of fractures in men with no previous osteoporotic fractures. The USPSTF identified the absence of randomized trials of primary fracture prevention in men with osteoporosis as a critical gap in the evidence.
Harms of detection and early intervention. The USPSTF found no new studies that described harms of screening for osteoporosis in men or women. Screening with DXA is associated with opportunity costs (time and effort required by patients and the health care system). Harms of drug therapies for osteoporosis depend on the specific medication used. The USPSTF found adequate evidence that the harms of bisphosphonates, the most commonly prescribed therapies, are no greater than small. Convincing evidence indicates that the harms of estrogen and selective estrogen receptor modulators are small to moderate.
USPSTF assessment. The USPSTF concludes that for women 65 years or older and younger women whose fracture risk is equal to or greater than that of a 65-year-old white woman with no additional risk factors, there is moderate certainty that the net benefit of screening for osteoporosis by using DXA is at least moderate.
The USPSTF concludes that, for men, evidence of the benefits of screening for osteoporosis is lacking, and the balance of benefits and harms cannot be determined.
Clinical Considerations
PATIENT POPULATION
This recommendation applies to older adults in the general U.S. population who do not have a history of an osteoporotic fracture, osteoporosis secondary to another condition, or other specific clinical indications for bone measurement testing. The USPSTF did not define a specific upper age limit for screening in women because the risk of fractures continues to increase with age, and treatment harms remain no greater than small. Clinicians should take into account the patient's remaining lifespan when deciding whether to screen patients with significant illness. In the Fracture Intervention Trial,1 the benefit of treatment emerged 18 to 24 months after initiation of treatment.
The quantity and quality of data on osteoporotic fracture risk other than hip fracture are much less for Asian, American Indian or Alaska Native, Hispanic, and black women than for white women. The USPSTF's recommendation to screen women 65 years or older for osteoporosis applies to all racial and ethnic groups because the harms of the screening tests are no greater than small, the consequences of failing to identify and treat women who have low bone mineral density (BMD) are considerable, and the optimal alternative age at which to screen nonwhite women is uncertain.
ASSESSMENT OF RISK
Multiple instruments to predict the risk of low BMD and fractures have been developed and validated for use in postmenopausal women, but few have been validated for use in men. To predict fracture risk, the area under the receiver-operating characteristic curve ranges from 0.48 to 0.89.2 Less complex instruments (i.e., those with fewer variables) seem to perform as well as more complex ones.3 The USPSTF found no studies that assessed the effect on patient outcomes of using risk prediction instruments alone or in combination with bone measurement tests.
The USPSTF used the World Health Organization's fracture risk assessment (FRAX) tool, available at http://www.shef.ac.uk/FRAX/, to estimate 10-year risks of fractures because this tool relies on easily obtainable clinical information, such as age, body mass index, parental fracture history, and tobacco and alcohol use; its development was supported by a broad international collaboration and extensively validated in two large U.S. cohorts; and it is freely accessible to clinicians and the public. The FRAX tool includes questions about previous DXA results but does not require this information to estimate fracture risk.
Based on the U.S. FRAX tool, a 65-year-old white woman with no other risk factors has a 9.3 percent 10-year risk of any osteoporotic fracture. White women between 50 and 64 years of age with equivalent or greater 10-year fracture risks based on specific risk factors include, but are not limited to, the following persons: (1) a 50-year-old current smoker with a body mass index less than 21 kg per m2, daily alcohol use, and parental fracture history; (2) a 55-year-old woman with a parental fracture history; (3) a 60-year-old woman with a body mass index less than 21 kg per m2 and daily alcohol use; and (4) a 60-year-old current smoker with daily alcohol use. The FRAX tool also predicts 10-year fracture risks for black, Asian, and Hispanic women in the United States. In general, estimated fracture risks in nonwhite women are lower than those for white women of the same age.
Although the USPSTF recommends using a 9.3 percent 10-year fracture risk threshold to screen women 50 to 64 years of age, clinicians also should consider each patient's values and preferences, and use clinical judgment when discussing screening with women in this age group. Menopausal status is one factor that may affect a decision about screening in this age group.
CONSIDERATIONS FOR PRACTICE REGARDING THE I STATEMENT
When deciding whether to screen men for osteoporosis, clinicians should consider the following factors:
Potential preventable burden. Bone measurement tests may potentially detect osteoporosis in a large number of men and prevent a substantial part of the burden of fractures and fracture-related illness in this population. The aging of the U.S. population is likely to increase this potentially preventable burden in future years.
Potential harms. Potential harms of screening men are likely to be small and consist primarily of opportunity costs.
Current practice. Routine screening of men currently is not a widespread practice.
Costs. Many additional DXA scanners may be required to screen sizeable populations of men for osteoporosis; DXA machines range in cost from $25,000 to $85,000.
Assuming that the relative benefits and harms of therapy in men are similar to those in women, the men most likely to benefit from screening would have 10-year risks of osteoporotic fracture equal to or greater than those of 65-year-old white women with no additional risk factors. However, current evidence is insufficient to assess the balance of benefits and harms of screening for osteoporosis in men.
SCREENING TESTS
The most commonly used bone measurement tests to screen for osteoporosis are DXA of the hip and lumbar spine, and quantitative ultrasonography of the calcaneus. Quantitative ultrasonography is less expensive and more portable than DXA and does not expose patients to ionizing radiation. Quantitative ultrasonography of the calcaneus predicts fractures of the femoral neck, hip, and spine as effectively as DXA. However, current diagnostic and treatment criteria for osteoporosis rely on DXA measurements only, and criteria based on quantitative ultrasonography or a combination of quantitative ultrasonography and DXA have not been defined.
SCREENING INTERVALS
The potential value of rescreening women whose initial screening test did not detect osteoporosis is to improve fracture risk prediction. A lack of evidence exists about optimal intervals for repeated screening and whether repeated screening is necessary in a woman with normal BMD. Because of limitations in the precision of testing, a minimum of two years may be needed to reliably measure a change in BMD; however, longer intervals may be necessary to improve fracture risk prediction. A prospective study of 4,124 women 65 years or older found that neither repeated BMD measurement nor the change in BMD after eight years was more predictive of subsequent fracture risk than the original measurement.4
TREATMENT
In addition to adequate calcium and vitamin D intake and weight-bearing exercise, multiple drug therapies are approved by the U.S. Food and Drug Administration to reduce fractures, including bisphosphonates, parathyroid hormone, raloxifene, and estrogen. The choice of therapy should be an individual one based on the patient's clinical situation and the tradeoff between benefits and harms. Clinicians should provide patient education on how to use drug therapies to minimize adverse effects. For example, esophageal irritation from bisphosphonate therapy can be reduced by taking the medication with a full glass of water and by not lying down for at least 30 minutes afterward.
OTHER APPROACHES TO PREVENTION
The USPSTF has updated its evidence review on fall prevention in older adults and plans to issue an updated recommendation; in future months, the USPSTF will also issue a separate statement on the preventive effects of vitamin D and calcium supplements on osteoporotic fractures. When complete, these documents will be made available at http://www.uspreventiveservicestaskforce.org.
USEFUL RESOURCES
The 10-year risk of osteoporotic fractures can be calculated for patients by using the FRAX tool and could help guide screening decisions for women younger than 65 years.
Summary guides for clinicians and patients on fracture prevention treatments for postmenopausal women with osteoporosis are available from the Agency for Healthcare Research and Quality at http://effectivehealthcare.ahrq.gov. The recommendations in these guides may differ from those of the USPSTF because they were based on a systematic review that pooled data from trials that included women with previous clinical fractures.
