Am Fam Physician. 2011;84(6):699-704
Author disclosure: No relevant financial affiliations to disclose.
Clinical Question
What therapies are effective for treating intermittent claudication?
Evidence-Based Answer
Supervised exercise programs are recommended to produce clinically significant improvements in walking distance in patients with stable intermittent claudication. (Strength of Recommendation [SOR]: A, based on systematic reviews of randomized controlled trials [RCTs].) Antiplatelet agents, statins, and pentoxifylline (Trental) can be prescribed to patients with claudication to improve walking distance. (SOR: B, based on systematic reviews of low-quality RCTs.) In most patients, percutaneous transluminal angioplasty (PTA) generally is not recommended. (SOR: B, based on systematic reviews of low-quality RCTs.) Direct comparison of effective therapies is not possible because of heterogeneous study populations and outcome measures.
Evidence Summary
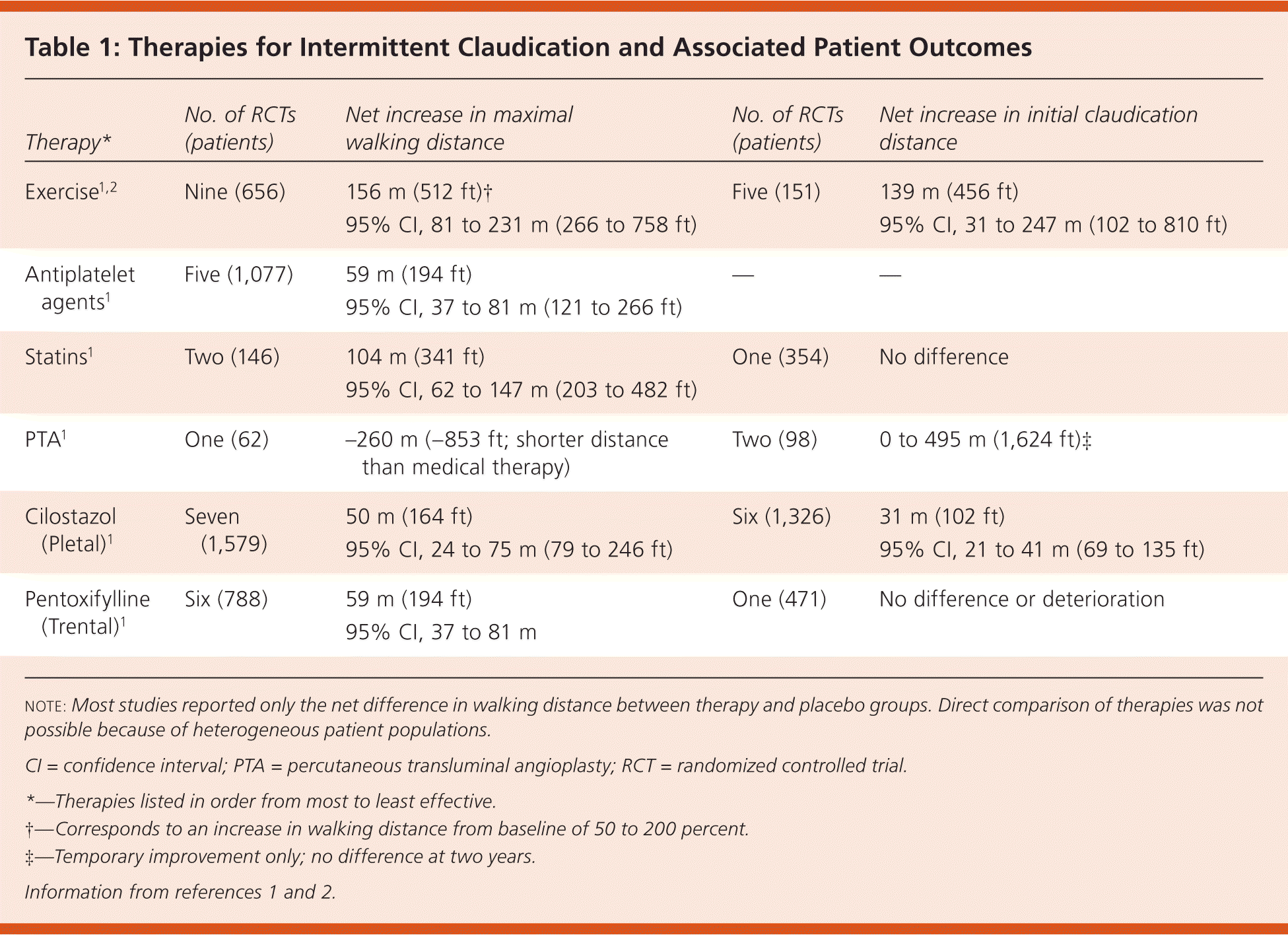
The studies evaluating the effectiveness of therapies for intermittent claudication symptoms included patients with various degrees of claudication and used heterogeneous outcome measures and statistical analyses, which precluded direct comparison. The claudication outcomes studied were initial claudication distance (i.e., the distance a patient can walk before the onset of claudication pain), absolute claudication distance (i.e., the distance a patient has walked when claudication pain prevents further walking), and maximal walking distance (i.e., the total distance walked, including stops to rest and recover). Therapies and outcome measures are listed in Table 1.1,2
| Therapy* | No. of RCTs (patients) | Net increase in maximal walking distance | No. of RCTs (patients) | Net increase in initial claudication distance |
|---|---|---|---|---|
| Exercise1,2 | Nine (656) | 156 m (512 ft)† 95% CI, 81 to 231 m (266 to 758 ft) | Five (151) | 139 m (456 ft) 95% CI, 31 to 247 m (102 to 810 ft) |
| Antiplatelet agents1 | Five (1,077) | 59 m (194 ft) 95% CI, 37 to 81 m (121 to 266 ft) | — | — |
| Statins1 | Two (146) | 104 m (341 ft) 95% CI, 62 to 147 m (203 to 482 ft) | One (354) | No difference |
| PTA1 | One (62) | –260 m (–853 ft; shorter distance than medical therapy) | Two (98) | 0 to 495 m (1,624 ft)‡ |
| Cilostazol (Pletal)1 | Seven (1,579) | 50 m (164 ft) 95% CI, 24 to 75 m (79 to 246 ft) | Six (1,326) | 31 m (102 ft) 95% CI, 21 to 41 m (69 to 135 ft) |
| Pentoxifylline (Trental)1 | Six (788) | 59 m (194 ft) 95% CI, 37 to 81 m | One (471) | No difference or deterioration |
EXERCISE PROGRAMS
The studies in two systematic reviews evaluated the effects of exercise in patients with stable chronic intermittent claudication who were randomized to participate in exercise programs or to receive usual care for periods of three to 24 months.1,2 The types of exercise included supervised sessions of strength training, pole striding (walking up an incline using modified ski poles), or upper or lower limb exercises. Programs lasted at least 30 to 40 minutes twice per week. In one systematic review, exercise improved walking time on a treadmill by about five minutes more than usual care, and produced improvements of 50 to 200 percent beyond baseline walking distances.2 Upper and lower limb exercises produced equivalent improvements. A third systematic review of eight RCTs (n = 319) found that supervised exercise improved walking distance by approximately 150 m (492 ft) compared with unsupervised exercise over three to 12 months.3
ANTIPLATELET AGENTS
Five RCTs in a systematic review (n = 1,077) evaluated the effects of antiplatelet therapy in patients with moderate intermittent claudication who took an antiplatelet agent over a five-to 12-month period.1 The results showed a 59-m (194-ft) increase in maximal walking distance (95% confidence interval, 37 to 81 m [121 to 266 ft]). Cilostazol (Pletal) at a dosage of 100 mg twice per day produced greater improvement than placebo at six to 24 weeks, but a dosage of 50 or 150 mg twice per day produced similar benefit. Cilostazol commonly led to adverse effects, including headache, diarrhea, and palpitations.1
STATINS
Two RCTs found that simvastatin (Zocor) improved maximal walking distance in patients with claudication, but the studies were statistically heterogeneous. A third RCT found that 80 mg of atorvastatin (Lipitor) per day improved initial claudication time by 40 seconds over 12 months but did not improve maximal walking time.1
PENTOXIFYLLINE
A systematic review of six RCTs found that pentoxifylline use improves maximal walking distances more than placebo. However, a large RCT found that pentoxifylline use did not change or worsen initial claudication distance.1
PERCUTANEOUS TRANSLUMINAL ANGIOPLASTY
One of two RCTs in a systematic review evaluating PTA in patients with mild to moderate claudication symptoms found short-term improvement, whereas the other RCT did not. Another RCT found that optimal medical therapy alone was superior to PTA plus medical therapy at two years. Low-quality and very low-quality RCTs found that routinely adding a stent to PTA produced greater maximal walking distance at 12 months than PTA alone.1
PROSTAGLANDIN E1
A systematic review (five very low-quality RCTs, n = 400) found that intravascular prostaglandins are unlikely to improve symptoms in patients with intermittent claudication. Prostaglandins also are associated with high rates of adverse effects, such as headache, vasodilation, diarrhea, and tachycardia.1
TREATMENTS WITH INCONCLUSIVE RESULTS
Recommendations from Others
The Eighth American College of Chest Physicians Conference on Antithrombotic and Thrombolytic Therapy recommends lifelong use of aspirin at a dosage of 75 to 100 mg per day in all patients with intermittent claudication. Clopidogrel (Plavix) is recommended in patients who cannot take aspirin. Cilostazol is recommended only in patients with disabling intermittent claudication who do not respond to risk factor modification and exercise, and who are not surgical candidates. The guidelines also recommend against the use of pentoxifylline, prostaglandins, and anticoagulants in patients with intermittent claudication.8
The American College of Cardiology/American Heart Association 2005 guidelines for the management of peripheral arterial disease recommend statins, antiplatelet therapy, risk factor modification, and supervised exercise training (30 to 45 minutes at least three times per week) for all patients with peripheral arterial disease. They recommend a trial of cilostazol for patients with lifestyle-limiting claudication. They also recommend surgical interventions in persons with functional disability from claudication symptoms that are unresponsive to exercise or pharmacotherapy but that have a reasonable likelihood of improvement.9