Am Fam Physician. 2011;84(7):805-814
A more recent article on bariatric surgery is available.
Related letter: Effects of Bariatric Surgeries on Obesity and Comorbidities
Patient information: See related handout on weight loss surgery, written by the authors of this article.
Author disclosure: No relevant financial affiliations to disclose.
Bariatric surgery procedures, including laparoscopic adjustable gastric banding, laparoscopic sleeve gastrectomy, and Roux-en-Y gastric bypass, result in an average weight loss of 50 percent of excess body weight. Remission of diabetes mellitus occurs in approximately 80 percent of patients after Roux-en-Y gastric bypass. Other obesity-related comorbidities are greatly reduced, and health-related quality of life improves. The Obesity Surgery Mortality Risk Score can help identify patients with increased mortality risk from bariatric surgery. Complications and adverse effects are lowest with laparoscopic surgery, and vary by procedure and presurgical risk. The Roux-en-Y procedure carries an increased risk of malabsorption sequelae, which can be minimized with standard nutritional supplementation. Outcomes are also influenced by the experience of the surgeon and surgical facility. Overall, these procedures have a mortality risk of less than 0.5 percent. Although there have been no long-term randomized controlled trials, existing studies show that bariatric surgery has a beneficial effect on mortality. The family physician is well positioned to care for obese patients by discussing surgery as an option for long-term weight loss. Counseling about the procedure options, risks and benefits of surgery, and the potential reduction in comorbid conditions is important. Patient selection, presurgical risk reduction, and postsurgical medical management, with nutrition and exercise support, are valuable roles for the family physician.
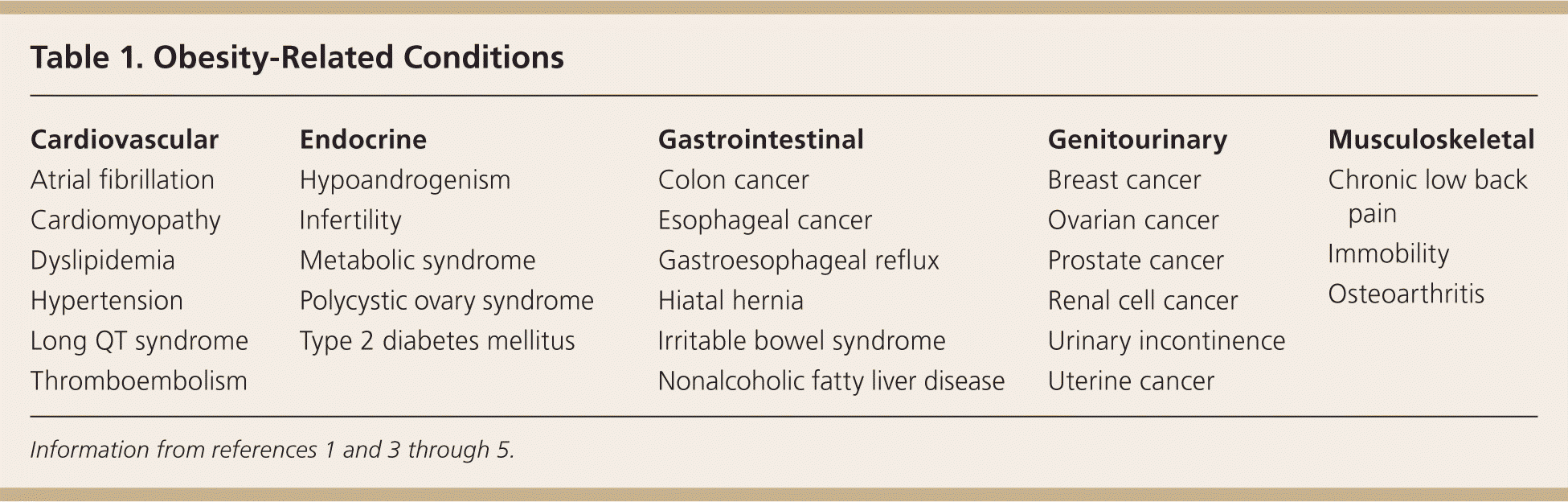
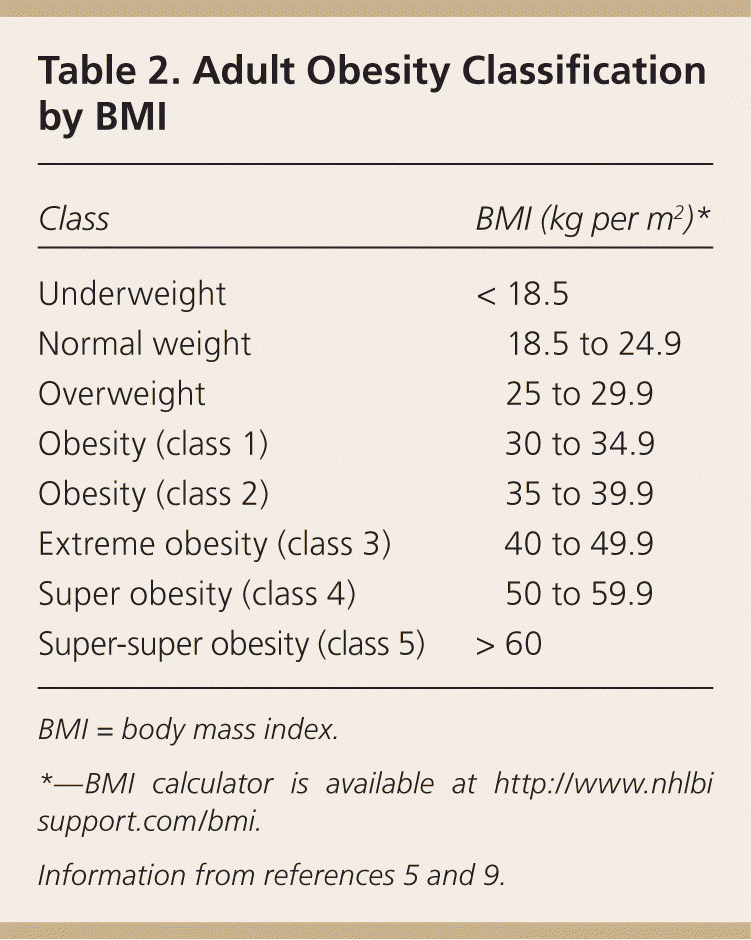
The prevalence of obesity has reached epidemic proportions. This disease has serious physical, psychological, and economic implications for patients and poses enormous challenges for the physicians caring for them.1 Approximately 68 percent of the U.S. adult population is overweight or obese.2,3 Obesity affects every organ system; the related pathologic processes create a tremendous health burden for patients (Table 1)1,3–5 and economic burden for the health care system. Obesity competes with smoking as the leading cause of preventable death in the United States.6,7 The U.S. Preventive Services Task Force recommends that physicians screen all adult patients for obesity and offer intensive counseling and behavioral interventions to promote sustained weight loss for obese adults.8 Body mass index approximates total body fat, although its accuracy varies by age, sex, race, and ethnic group (Table 2).5,9 Intensive lifestyle intervention can result in significant weight loss (10 percent or greater) in obese patients and can be initiated by the family physician. However, adherence rates are low,10 it is time-prohibitive, and it may be considered ineffectual over the long term.11 Surgical treatment of obesity results in greater weight loss and greater reduction in comorbid conditions compared with traditional therapy.1
| Clinical recommendation | Evidence rating | References |
|---|---|---|
| Bariatric surgery results in greater weight loss than conventional weight-loss programs for persons in all classes of obesity. | A | 1 |
| The use of a clinical rule (Table 5) can predict the risk of perioperative mortality in patients undergoing bariatric surgery. | C | 20–22 |
| Bariatric surgery is highly effective in treating obesity-related comorbidities, including diabetes mellitus, hyperlipidemia, and hypertension. | A | 27, 36 |
| Individual outcomes are improved by choosing an experienced bariatric surgeon and a high-volume surgical center. | C | 17, 31–33 |
| Bariatric surgery may reduce disease-related mortality by up to 40 percent. | B | 47 |
| Cardiovascular | Endocrine | Gastrointestinal | Genitourinary | Musculoskeletal |
|---|---|---|---|---|
|
|
|
|
|
| Class | BMI (kg per m2)* | |
|---|---|---|
| Underweight | < 18.5 | |
| Normal weight | 18.5 to 24.9 | |
| Overweight | 25 to 29.9 | |
| Obesity (class 1) | 30 to 34.9 | |
| Obesity (class 2) | 35 to 39.9 | |
| Extreme obesity (class 3) | 40 to 49.9 | |
| Super obesity (class 4) | 50 to 59.9 | |
| Super-super obesity (class 5) | > 60 | |
Indications and Eligibility
The number of bariatric procedures performed in the United States increased from 13,365 in 1998 to more than 200,000 in 2008.12 Bariatric surgery is usually considered when other weight loss efforts have failed. Eligibility criteria were established by the 1991 National Institutes of Health Consensus Development Conference Panel and continue to be the most widely accepted criteria.9 Selection and exclusion criteria are listed in Table 3.5,13–15 Recent data suggest that patients with diabetes mellitus and a body mass index of 30 to 35 kg per m2 may also be reasonable candidates for bariatric surgery.13–15

| Selection criteria |
| Able to adhere to postoperative care (e.g., follow-up visits and tests, medical management, use of dietary supplements) |
| BMI ≥ 40 kg per m2 |
| BMI ≥ 35 kg per m2 with obesity-related comorbidity* |
| Previous failed nonsurgical attempts at weight reduction, including nonprofessional programs (e.g., Weight Watchers) |
| Exclusion criteria |
| Cardiopulmonary disease that would make the risk prohibitive |
| Current drug or alcohol abuse |
| Lack of comprehension of risks, benefits, expected outcomes, alternatives, and required lifestyle changes |
| Reversible endocrine or other disorders that can cause obesity |
| Uncontrolled severe psychiatric illness |
To qualify for Medicare and Medicaid reimbursement, patients must be referred to a facility that is a designated center of excellence by the American Society for Metabolic and Bariatric Surgery, or that is accredited by the American College of Surgeons Bariatric Surgery Center Network.16 Many private insurers also apply these criteria.
Preoperative Considerations
Evaluation of the surgical candidate is often conducted by a multidisciplinary team with expertise in nutrition, psychology/psychiatry, surgery, and medicine (Table 4).17 Surgeon preference and the requirements of third-party payers determine the scope of presurgical evaluation and the role of each team member. Consultation with a dietitian or nutritionist is usually required. The surgeon obtains a comprehensive obesity-focused history that includes current dietary habits, activity and exercise patterns, and all previous weight loss efforts. Psychiatric diagnoses are common among patients considering weight loss surgery, but there are no evidence- or consensus-based guidelines to identify patients whose psychological status make them inappropriate candidates for bariatric surgery.18 When psychiatric diagnoses are suspected, further evaluation by a mental health professional may be warranted.

| Blood chemistry profile, including liver function tests |
| Chest radiography |
| Coagulation profile |
| Complete blood count |
| Electrocardiography |
| Lipid profile |
| Measurement of fasting blood glucose; A1C; ferritin; thyroid-stimulating hormone, 25-hydroxyvitamin D; and vitamin B1, B6, and B12 levels |
| Nutrition consultation |
| Psychological evaluation |
| Subspecialist consultation as indicated (e.g., endoscopy, sleep study) |
| Urinalysis |
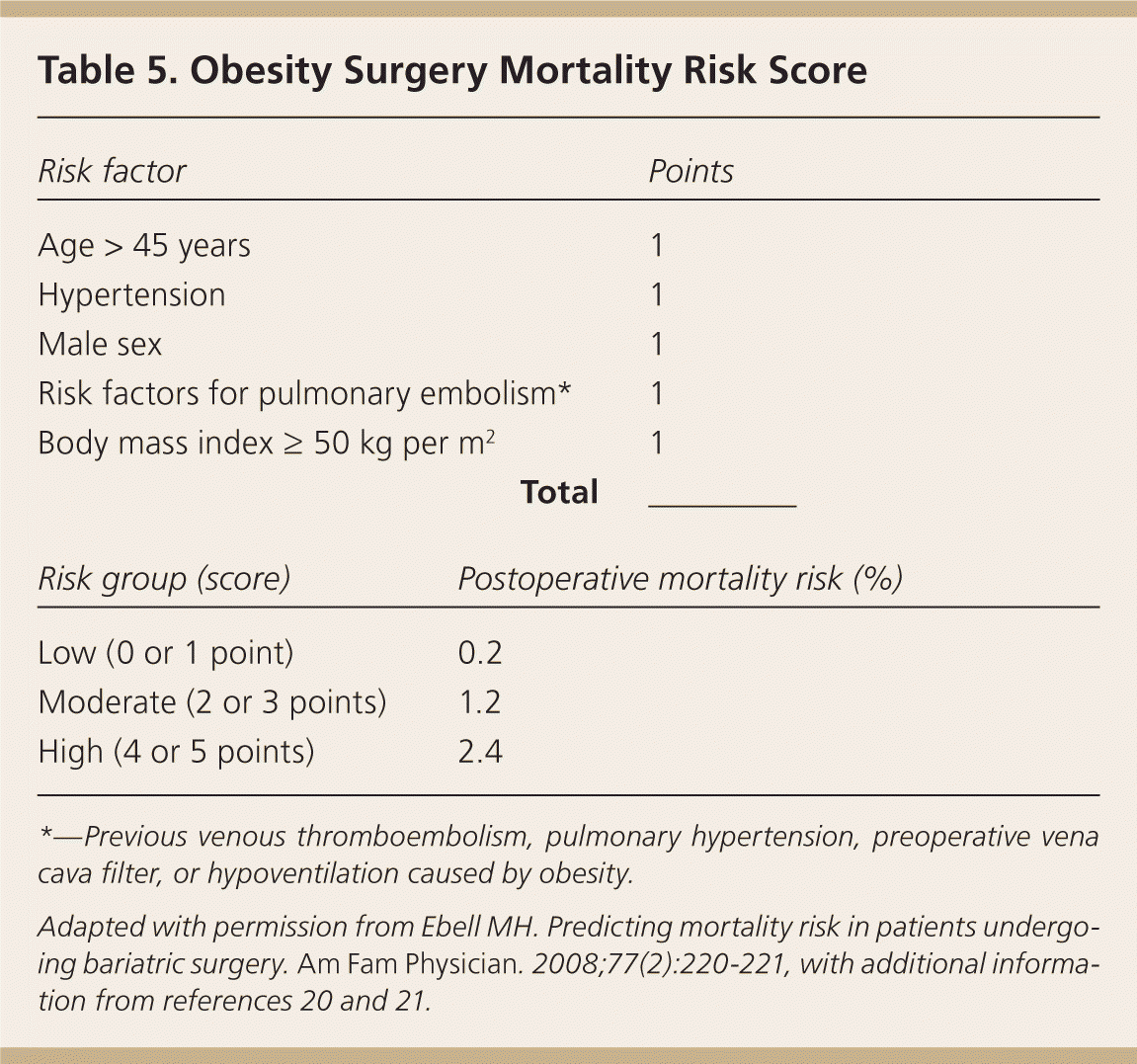
Six months of medical weight management is commonly required before surgical approval is granted; this may be provided by the family physician.19 Weight-related comorbidities that may increase surgical risk should be aggressively managed. The Obesity Surgery Mortality Risk Score allows for prediction of postoperative mortality (Table 5).20–22 Numerous medications contribute to weight gain, and alternatives should be sought whenever possible (Table 6).23,24 Findings suggestive of metabolic disorders (e.g., acanthosis nigricans) and signs of secondary causes of obesity (e.g., Cushing syndrome) should be identified. Further evaluation should be guided by the history and physical examination, and may include additional laboratory studies or procedures such as polysomnography for obstructive sleep apnea. Some surgeons require presurgical weight loss, but there is little evidence to support this requirement except when reduction of liver volume would significantly improve the technical aspects of surgery.17
| Risk factor | Points |
|---|---|
| Age > 45 years | 1 |
| Hypertension | 1 |
| Male sex | 1 |
| Risk factors for pulmonary embolism* | 1 |
| Body mass index ≥ 50 kg per m2 | 1 |
| Total ________ |
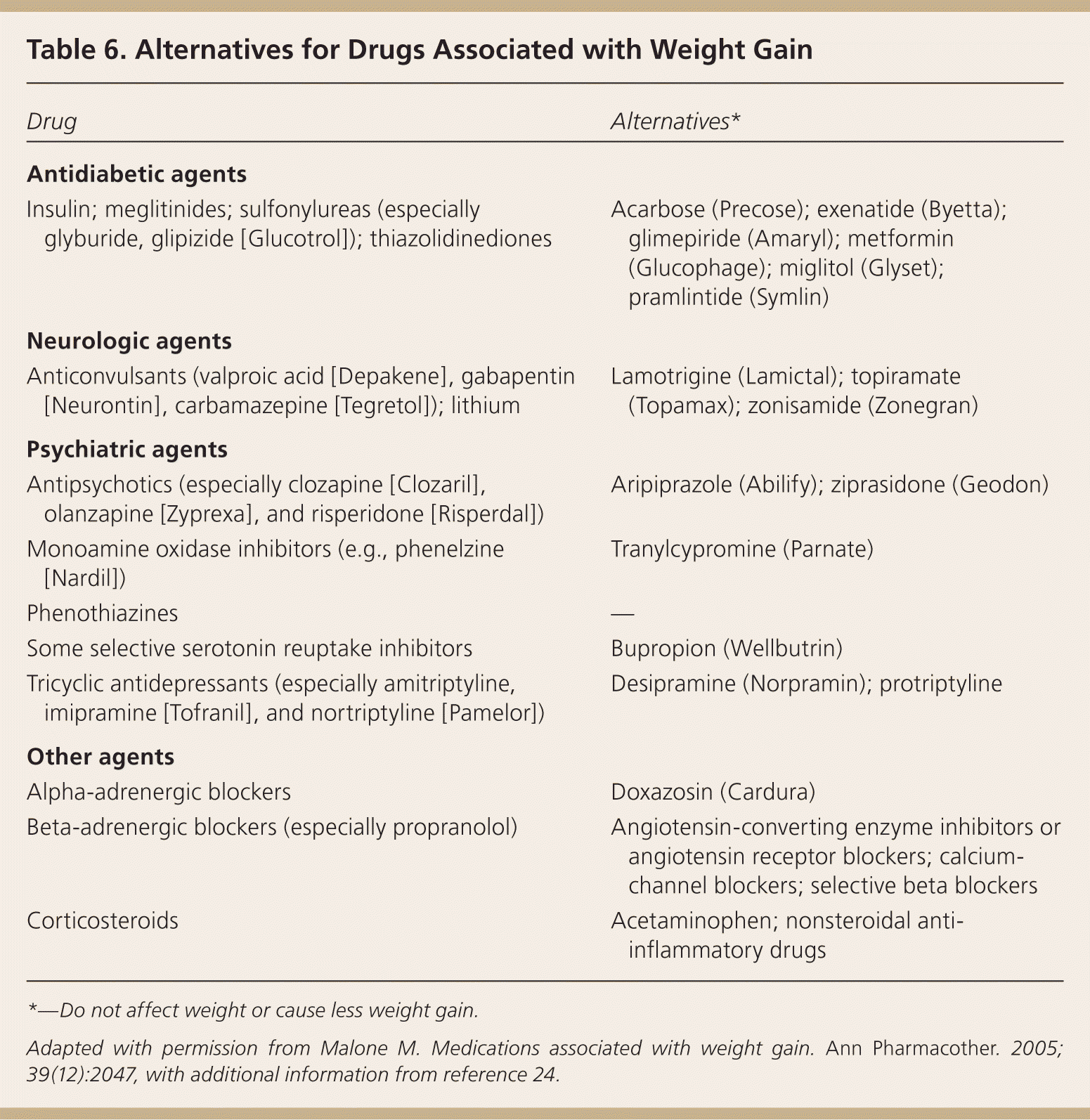
| Drug | Alternatives* |
|---|---|
| Antidiabetic agents | |
| Insulin; meglitinides; sulfonylureas (especially glyburide, glipizide [Glucotrol]); thiazolidinediones | Acarbose (Precose); exenatide (Byetta); glimepiride (Amaryl); metformin (Glucophage); miglitol (Glyset); pramlintide (Symlin) |
| Neurologic agents | |
| Anticonvulsants (valproic acid [Depakene], gabapentin [Neurontin], carbamazepine [Tegretol]); lithium | Lamotrigine (Lamictal); topiramate (Topamax); zonisamide (Zonegran) |
| Psychiatric agents | |
| Antipsychotics (especially clozapine [Clozaril], olanzapine [Zyprexa], and risperidone [Risperdal]) | Aripiprazole (Abilify); ziprasidone (Geodon) |
| Monoamine oxidase inhibitors (e.g., phenelzine [Nardil]) | Tranylcypromine (Parnate) |
| Phenothiazines | — |
| Some selective serotonin reuptake inhibitors | Bupropion (Wellbutrin) |
| Tricyclic antidepressants (especially amitriptyline, imipramine [Tofranil], and nortriptyline [Pamelor]) | Desipramine (Norpramin); protriptyline |
| Other agents | |
| Alpha-adrenergic blockers | Doxazosin (Cardura) |
| Beta-adrenergic blockers (especially propranolol) | Angiotensin-converting enzyme inhibitors or angiotensin receptor blockers; calcium-channel blockers; selective beta blockers |
| Corticosteroids | Acetaminophen; nonsteroidal anti-inflammatory drugs |
Pathophysiology
Notable improvements in blood glucose levels occur in patients after bariatric surgery, even before significant weight loss has occurred. In addition to decreased caloric intake, multiple mechanisms seem to contribute to the dramatic improvement of diabetes after procedures that bypass normal anatomy. Levels of glucagon-like peptide-1 and peptide YY, which are secreted by intestinal L cells, increase after gastric bypass procedures. Glucagon-like peptide-1 enhances insulin secretion, whereas peptide YY increases satiety and delays gastric emptying through receptors in the central and peripheral nervous system. Ghrelin, which is secreted primarily by the gastric fundus and proximal small intestine, acts via the hypothalamus to stimulate appetite and suppress energy expenditure and fat catabolism. Bypass procedures seem to reduce the secretion of ghrelin and reduce appetite. A complex neuroendocrine system involving neurotransmitters and hormones of the gut, brain, central and peripheral nervous systems, and adipocytes interact to regulate energy homeostasis.25,26 Remission rates of type 2 diabetes after bariatric surgery may be as high as 70 to 80 percent.27
Choice of Procedure
More than 90 percent of bariatric surgeries are performed laparoscopically; this method is preferred to open procedures.5 Laparoscopic procedures are as effective as open procedures and result in fewer wound complications, shorter hospital stays, and more rapid recovery.
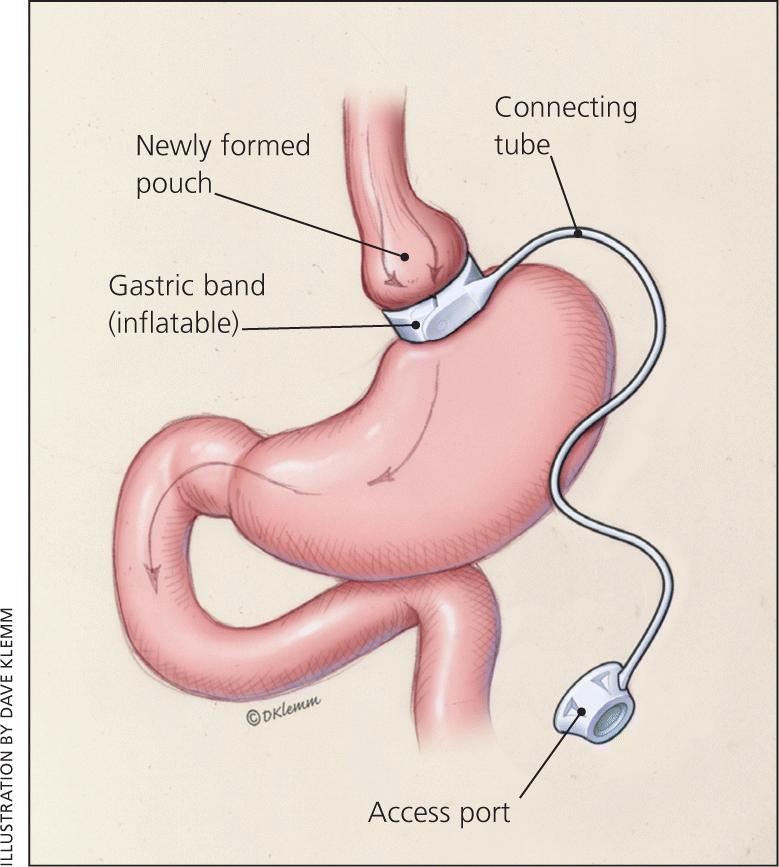
Three procedures are commonly performed: laparoscopic adjustable gastric banding (LAGB), laparoscopic sleeve gastrectomy (LSG), and Roux-en-Y gastric bypass (RYGB). In LAGB, a hollow, flexible silicone band is placed around the upper stomach, which causes a restrictive effect, reduces stomach capacity, and causes rapid feelings of satiety. The band is tightened by injecting saline into the band via a subcutaneous port, located just inferior to the sternum or lateral to the umbilicus (Figure 1).
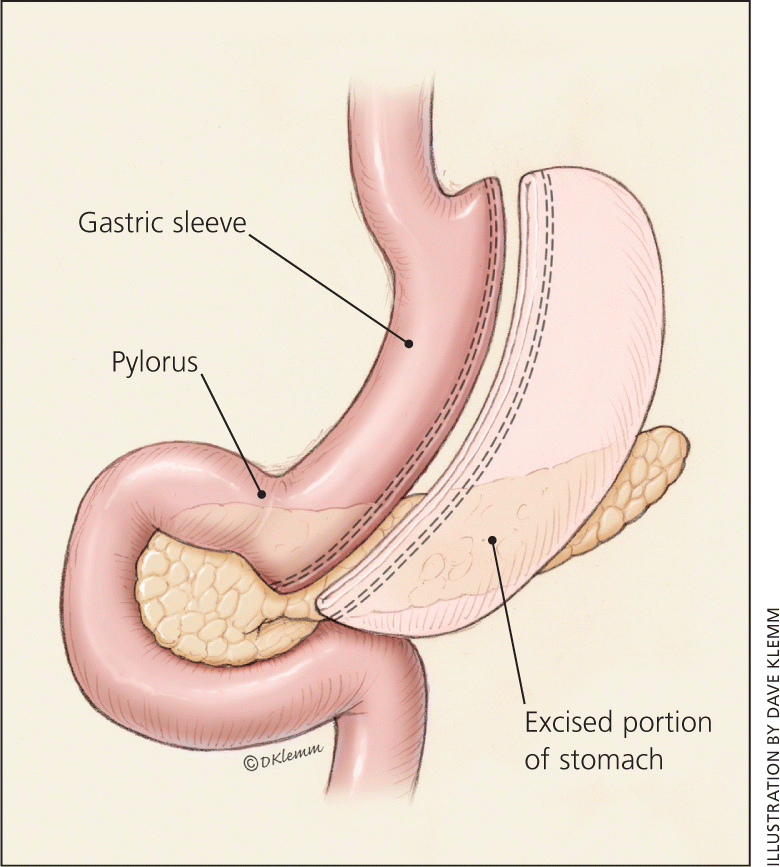
The LSG procedure resects most of the body and all of the fundus of the stomach, creating a long, narrow, tubular stomach (Figure 2). This procedure was first used as an initial step before a malabsorptive procedure in very high-risk patients, but is now approved as a primary stand-alone procedure.28,29
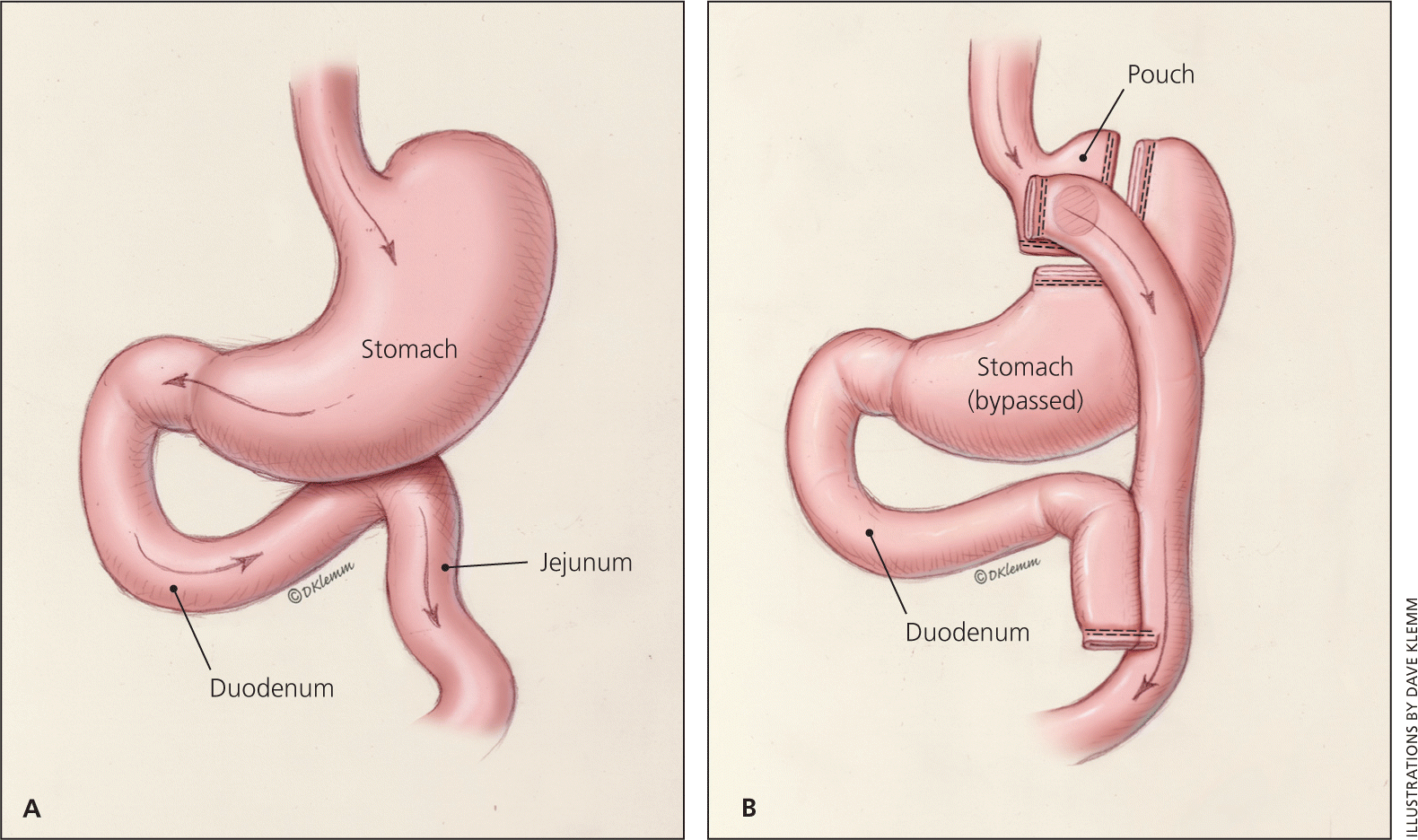
In RYGB, a small gastric pouch is formed by dividing the upper stomach and joining it with the resected end of jejunum, so that food bypasses the stomach and upper small bowel, thereby restricting the size of the stomach and causing some malabsorption (Figure 3). RYGB may be a better choice in more obese patients and in those with type 2 diabetes.13,30 RYGB is the most common procedure (51 percent) performed in the United States and Canada, followed by LAGB (44 percent).12 The biliopancreatic diversion, with or without duodenal switch, is an older procedure that is no longer commonly performed.5
Early Management
The bariatric surgeon generally provides early postoperative management, including the progression of diet from clear liquids to regular food over the first four to eight weeks. This period varies depending on the surgeon and patient. Patients who undergo RYGB may require liquid formulations of medications and vitamins, whereas these formulations are not necessary for patients who undergo LAGB and LSG. The patient's medical conditions are often managed by the family physician in the weeks following surgery. In particular, diabetes must be aggressively monitored; medication requirements often change quickly after surgery and before significant weight loss occurs. Dumping syndrome, with symptoms of abdominal pain, nausea, diarrhea, light-headedness, flushing, and tachycardia, occurs in up to 70 percent of patients after RYGB.5 These symptoms can be largely eliminated by avoiding simple sugars; eating small, frequent meals; and increasing protein intake.5
Long-Term Management
The family physician should have the primary role in the comprehensive long-term care of patients after bariatric surgery. Traditional weight loss lifestyle changes still need to occur. The multidisciplinary team can be invaluable at this time.34 Patients must have regular follow-up for band adjustments after LAGB. The adjustments are often performed by the surgeon, directly or under fluoroscopic guidance, although in some geographic areas, a physician assistant or primary care physician provides this service.
After bariatric surgery, patients are encouraged to begin each meal with protein to ensure adequate intake (approximately 80 g per day) and minimize the loss of lean body mass. Vegetable consumption should also be encouraged. Food intolerances are patient specific, but very dry foods, breads, and fibrous vegetables are often problematic.35 Patients should be advised to eat slowly and chew thoroughly. Fluids should be avoided for 15 to 30 minutes before, during, and after meals because ingested food will pass easily through the pouch opening if it is mixed with fluid, and the sensation of fullness will not be achieved.5 Patients are often advised to avoid drinking with straws because of increased air intake, and to avoid carbonated beverages because they may expand the pouch; however, there is little evidence to support these recommendations. Cold intolerance, hair loss, and fatigue are common but tend to diminish rapidly as weight loss stabilizes. Women should avoid becoming pregnant for 18 months after bariatric surgery.5
Quarterly assessment of nutritional status and supplementation needs, food intolerances, and symptoms should occur for the first year after bariatric surgery. A variety of micronutrient deficiencies have been identified after malabsorption procedures, and even after some restrictive procedures because of decreased capacity for food intake. Vitamin supplementation will be required throughout the patient's lifetime, and annual metabolic and nutritional monitoring is recommended, although no standard exists (Table 7).5,35 Vitamin and mineral deficiencies may not become apparent for many years following bariatric surgery.
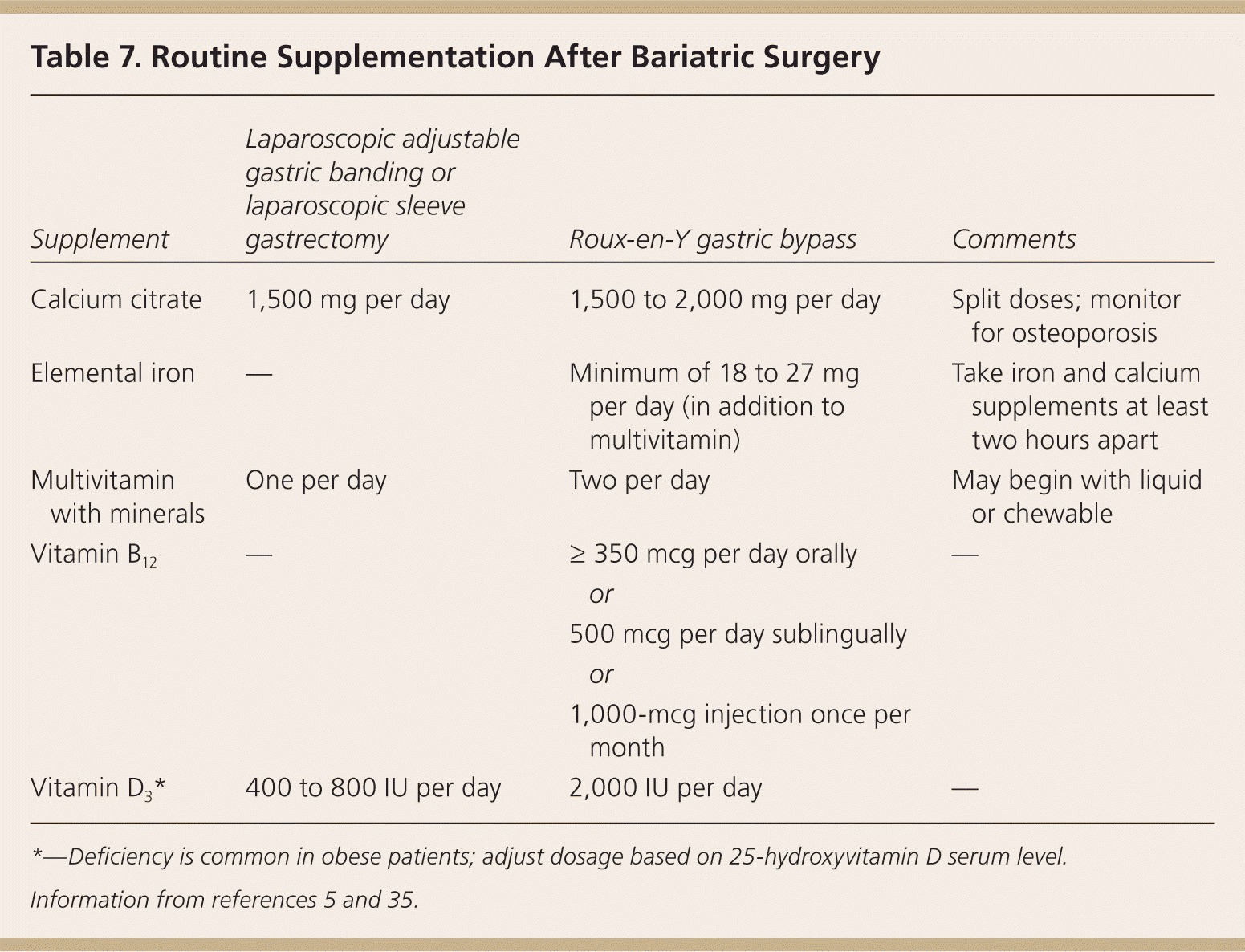
| Supplement | Laparoscopic adjustable gastric banding or laparoscopic sleeve gastrectomy | Roux-en-Y gastric bypass | Comments | |
|---|---|---|---|---|
| Calcium citrate | 1,500 mg per day | 1,500 to 2,000 mg per day | Split doses; monitor for osteoporosis | |
| Elemental iron | — | Minimum of 18 to 27 mg per day (in addition to multivitamin) | Take iron and calcium supplements at least two hours apart | |
| Multivitamin with minerals | One per day | Two per day | May begin with liquid or chewable | |
| Vitamin B12 | — | ≥ 350 mcg per day orally | — | |
| or | ||||
| 500 mcg per day sublingually | ||||
| or | ||||
| 1,000-mcg injection once per month | ||||
| Vitamin D3* | 400 to 800 IU per day | 2,000 IU per day | — | |
Outcomes
In general, RYGB seems to lead to the greatest weight loss (up to 10 lb [4.5 kg] per month) during the first one to two postsurgical years, followed by LSG and LAGB. It is unclear if there is a significant long-term difference in weight loss or maintenance (Table 8).5,14,27,36–41 The surgical literature typically expresses weight loss as percentage of excess body weight lost. Successful weight loss after bariatric surgery is considered at least 50 percent of excess body weight, whereas successful medical weight loss is considered 5 to 10 percent of baseline weight.42 After bariatric surgery, many patients maintain a long-term (eight- to 10-year) weight loss of greater than 50 percent of excess body weight.43 Hyperlipidemia, diabetes, hypertension, and most other obesity-related conditions are significantly improved after any surgical procedure.29,44 Patients' perception of well-being, social function, body image, and self-confidence also improve after bariatric surgery.45,46 In addition to weight loss, the improvement in comorbidities and in overall health is important in evaluating the success of bariatric surgery.
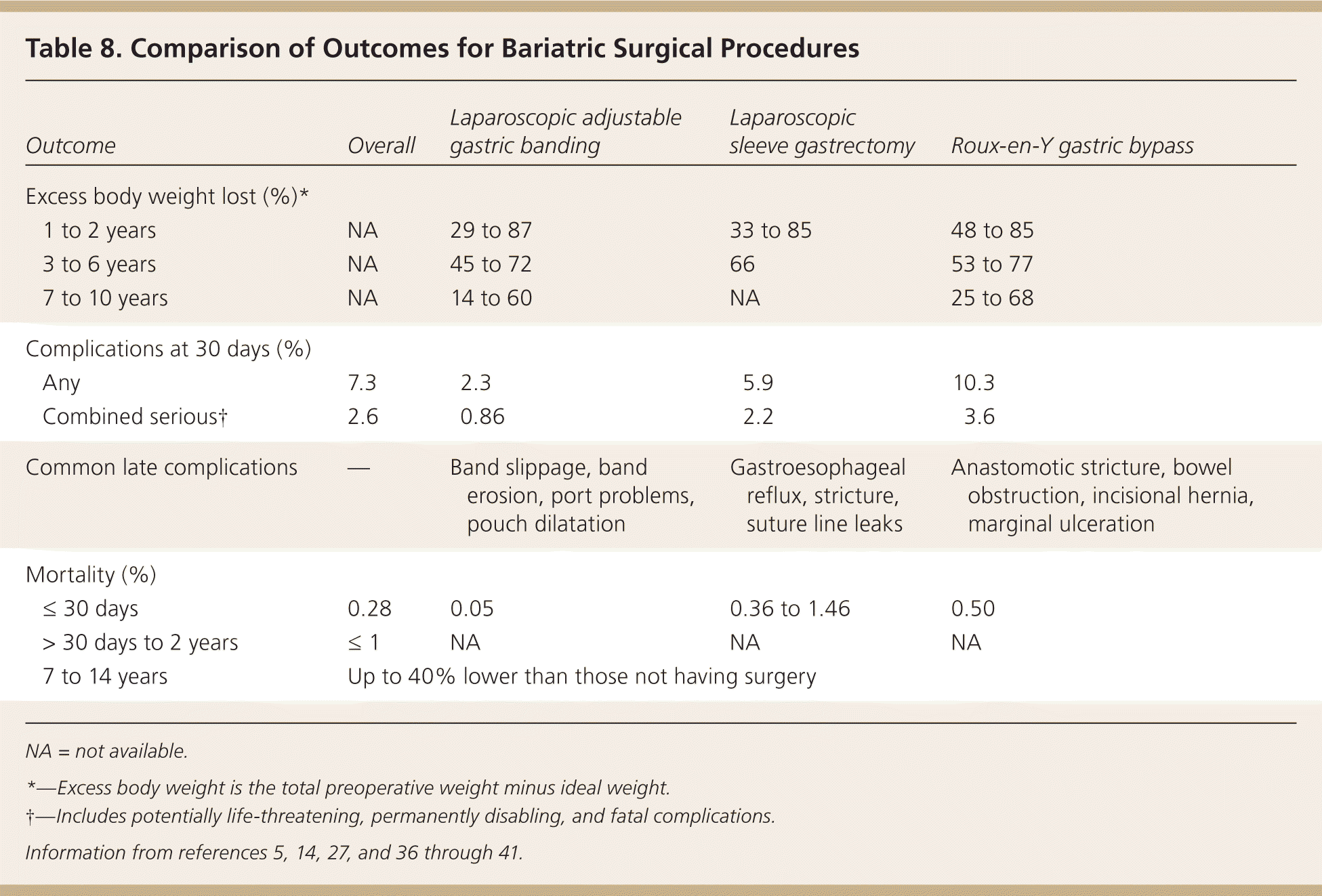
| Outcome | Overall | Laparoscopic adjustable gastric banding | Laparoscopic sleeve gastrectomy | Roux-en-Y gastric bypass | |
|---|---|---|---|---|---|
| Excess body weight lost (%)* | |||||
| 1 to 2 years | NA | 29 to 87 | 33 to 85 | 48 to 85 | |
| 3 to 6 years | NA | 45 to 72 | 66 | 53 to 77 | |
| 7 to 10 years | NA | 14 to 60 | NA | 25 to 68 | |
| Complications at 30 days (%) | |||||
| Any | 7.3 | 2.3 | 5.9 | 10.3 | |
| Combined serious† | 2.6 | 0.86 | 2.2 | 3.6 | |
| Common late complications | — | Band slippage, band erosion, port problems, pouch dilatation | Gastroesophageal reflux, stricture, suture line leaks | Anastomotic stricture, bowel obstruction, incisional hernia, marginal ulceration | |
| Mortality (%) | |||||
| ≤ 30 days | 0.28 | 0.05 | 0.36 to 1.46 | 0.50 | |
| > 30 days to 2 years | ≤ 1 | NA | NA | NA | |
| 7 to 14 years | Up to 40% lower than those not having surgery | ||||
Complication rates are difficult to assess across different studies because of the evolution of surgical procedures, laparoscopic versus open technique, categorization of short- versus long-term sequelae, and the difference in presurgical risk among patient populations. To better define current morbidity and mortality outcomes, the National Institutes of Health initiated the Longitudinal Assessment of Bariatric Surgery Consortium, which is conducting prospective, multicenter, observational cohort studies using standardized techniques to assess the safety and clinical response of bariatric surgery.39
Data about the effect of long-term weight loss on mortality has been conflicting, particularly when comparing the risks of bariatric surgery against the benefits of weight loss. In a retrospective cohort study of almost 8,000 patients undergoing bariatric surgery, mortality from disease, including cardiovascular disease and cancer, decreased by 40 percent compared with the control group.47 However, death from accidents and suicide increased 1.58-fold in the surgical group; it is not clear if undiagnosed presurgical psychological disorders were a factor. Decision modeling suggests that bariatric surgery increases life expectancy by an average of three years; however, a very high surgical risk may negate the potential benefit. This reinforces the need for careful patient selection and presurgical reduction of modifiable risks.22,48 There are no long-term randomized controlled trials evaluating the impact of bariatric surgery on mortality, but observational studies (including case-control studies47 and a large nonrandomized prospective observational cohort49) found that bariatric surgery has a beneficial effect on mortality.
Cost
It is estimated that obesity accounts for 10 percent of all medical spending.50 In 2006, per capita medical spending for obese persons was $1,429 (42 percent) higher than normal-weight persons, for an estimated $147 billion per year direct cost to the health care system.50 It is estimated that costs associated with laparoscopic surgery are fully recovered by third party payers after 25 months.51 The overall decrease in comorbid conditions, prescription drug use, hospital stays, and physician visits rapidly outweighs the initial cost of surgery.25
Data Sources: The following sources were reviewed: the Agency for Healthcare Research and Quality, the Cochrane Database of Systematic Reviews, Essential Evidence Plus, the U.S. Preventive Services Task Force, DynaMed, and evidence-based practice guidelines from the Society of American Gastrointestinal and Endoscopic Surgeons. References from key articles were searched, as were references provided by AFP in original correspondence. In addition, an OVID search was performed using the key words obesity, bariatric, weight loss, and surgery. Search dates: April 2010 and December 2010.