
Am Fam Physician. 2011;84(8):895-902
A more recent article on Alzheimer disease is available.
Author disclosure: No relevant financial affiliations to disclose.
As the proportion of persons in the United States older than 65 years increases, the prevalence of dementia will increase as well. Risk factors for dementia include age, family history of dementia, apolipoprotein E4 genotype, cardiovascular comorbidities, chronic anticholinergic use, and lower educational level. Patient history, physical examination, functional assessment, cognitive testing, laboratory studies, and imaging studies are used to assess a patient with suspected dementia. A two-visit approach is time-effective for primary care physicians in a busy outpatient setting. During the first visit, the physician should administer a screening test such as the verbal fluency test, the Mini-Cognitive Assessment Instrument, or the Sweet 16. These tests have high sensitivity and specificity for detecting dementia, and can be completed in as little as 60 seconds. If the screening test result is abnormal or clinical suspicion of another disease is present, appropriate laboratory and imaging tests should be ordered, and the patient should return for additional cognitive testing. A second visit should include a Mini-Mental State Examination, Geriatric Depression Scale, and verbal fluency and clock drawing tests, if not previously completed.
The proportion of persons in the United States older than 65 years will grow from 12 percent to more than 20 percent in the next 20 years, and this increase will bring a higher prevalence of dementia.1 Early identification of cognitive impairment can help patients and their physicians to enact appropriate advance care planning, identify comorbidities and secondary causes of cognitive dysfunction, and discuss initiation of medical therapy. The U.S Preventive Services Task Force recommends cognitive assessment if cognitive impairment or deterioration is suspected.2 However, primary care physicians often cite time constraints as a barrier to performing this assessment; as a result, dementia may go unrecognized.3 This article addresses the assessment of suspected dementia, including a review of practical tools for evaluating cognitive impairment and geriatric depression.
| Clinical recommendation | Evidence rating | References |
|---|---|---|
| For patients with suspected dementia, quick screening tests, such as a verbal fluency test and the Mini-Cognitive Assessment Instrument, should be performed to determine whether further evaluation is warranted. | C | 13, 14 |
| Formal cognitive testing should be performed in patients with abnormal results on initial dementia screening. | C | 27 |
| Routine blood work (i.e., complete blood count; complete metabolic panel; and measurement of thyroid-stimulating hormone, vitamin B12, folate, and calcium levels) should be performed in patients with suspected dementia. | C | 27, 28 |
| Additional testing (e.g., neuroimaging, cerebrospinal fluid analysis, human immunodeficiency virus testing, Lyme titer, rapid plasma reagin test) can be performed in patients with suspected dementia and specific risk factors or symptoms. | C | 27 |
Epidemiology and Risk Factors
After 65 years of age, the lifetime risk of developing dementia is approximately 17 to 20 percent; 70 percent of patients with dementia have Alzheimer disease, 17 percent have vascular dementia, and 13 percent have a combination of dementia with Lewy bodies, Parkinson-related dementia, alcoholic dementia, or frontal lobe dementia.4,5 The transitional state between normal cognition and early Alzheimer disease is called mild cognitive impairment, which is defined as memory impairment without meeting criteria for dementia. Each year, 10 to 15 percent of patients with mild cognitive impairment develop Alzheimer disease.6 Alzheimer disease affects 5.3 million Americans, and is the sixth leading cause of death.4 Median survival time after diagnosis of dementia is 4.5 years.7
Risk factors for dementia include age, family history of dementia, apolipoprotein E4 genotype, cardiovascular comorbidities, chronic anticholinergic use, and lower educational level.8–10 The greatest risk factor for dementia is increasing age. In persons 71 to 79 years of age, the prevalence is approximately 5 percent, increasing to 37 percent in persons older than 90 years.5 Having a college education has been shown to delay cognitive dysfunction by two years, compared with having less education.10 The presence of the apolipoprotein E4 genotype can increase the risk of dementia two- to 10-fold, and chronic anticholinergic use is associated with a somewhat increased risk (hazard ratio = 1.65).5,9
Diagnostic Guidelines
The National Institute on Aging and the Alzheimer's Association have released guidelines for clinical diagnosis of dementia and mild cognitive impairment. In brief, dementia can be diagnosed if cognitive or behavioral symptoms interfere with the patient's ability to function at work or socially, if there is a decline from previous functioning, and if cognitive or behavioral impairments are detected through a combination of history and cognitive assessment.11 The cognitive or behavioral impairments must be present in at least two of the following domains: ability to recall new information, reasoning, visuospatial ability, language, and personality.11 Mild cognitive impairment is defined as impairment in at least one of these domains; concern about cognition as expressed by the patient, an informant, or the physician; and preservation of independence and the ability to work.12
Initial History and Functional Assessment
When dementia is suspected, physicians should obtain a history from the patient and from a family member or caregiver, because patients with dementia often do not have insight into their deficits. The history should include specifics of cognitive deficit, time of onset, and speed of progression. It is imperative to assess the extent of impairment in instrumental activities of daily living, which include managing money and medications, shopping, housekeeping, cooking, and transportation. In the early stages of dementia, instrumental activities of daily living that require calculation and planning, such as balancing a check-book or filling a pillbox, are often the first to become impaired. Basic activities of daily living, such as dressing, eating, toileting, and grooming, are generally intact in early dementia and do not become impaired until later in the disease progression.
Other notable indicators of dementia include a history of visual hallucinations in patients who have dementia with Lewy bodies, inappropriate disinhibition in patients with frontotemporal dementia, classic symptoms of Parkinson disease in patients with Parkinson-related dementia, and alcohol abuse in patients with alcoholic dementia. Psychoactive drugs, such as benzodiazepines, can cause confusion in older persons. Hypothyroidism and depression commonly cause cognitive impairment, and patients should be screened for signs and symptoms, such as weight change, sleep disturbance, and mood instability. In patients with recent hospitalization or underlying psychiatric disorder, delirium should be considered. Finally, educational level and native language should be assessed, because these can influence scores on several cognitive tests.
Although the physical examination is not usually affected in patients with Alzheimer disease, abnormalities can give clues about less common types of dementia. Focal deficits from a previous stroke are common in patients with vascular dementia. Parkinsonism is seen in patients during the later stages of dementia with Lewy bodies. Table 1 lists key findings from the patient history and physical examination that may accompany cognitive dysfunction, and the suggested diagnoses.
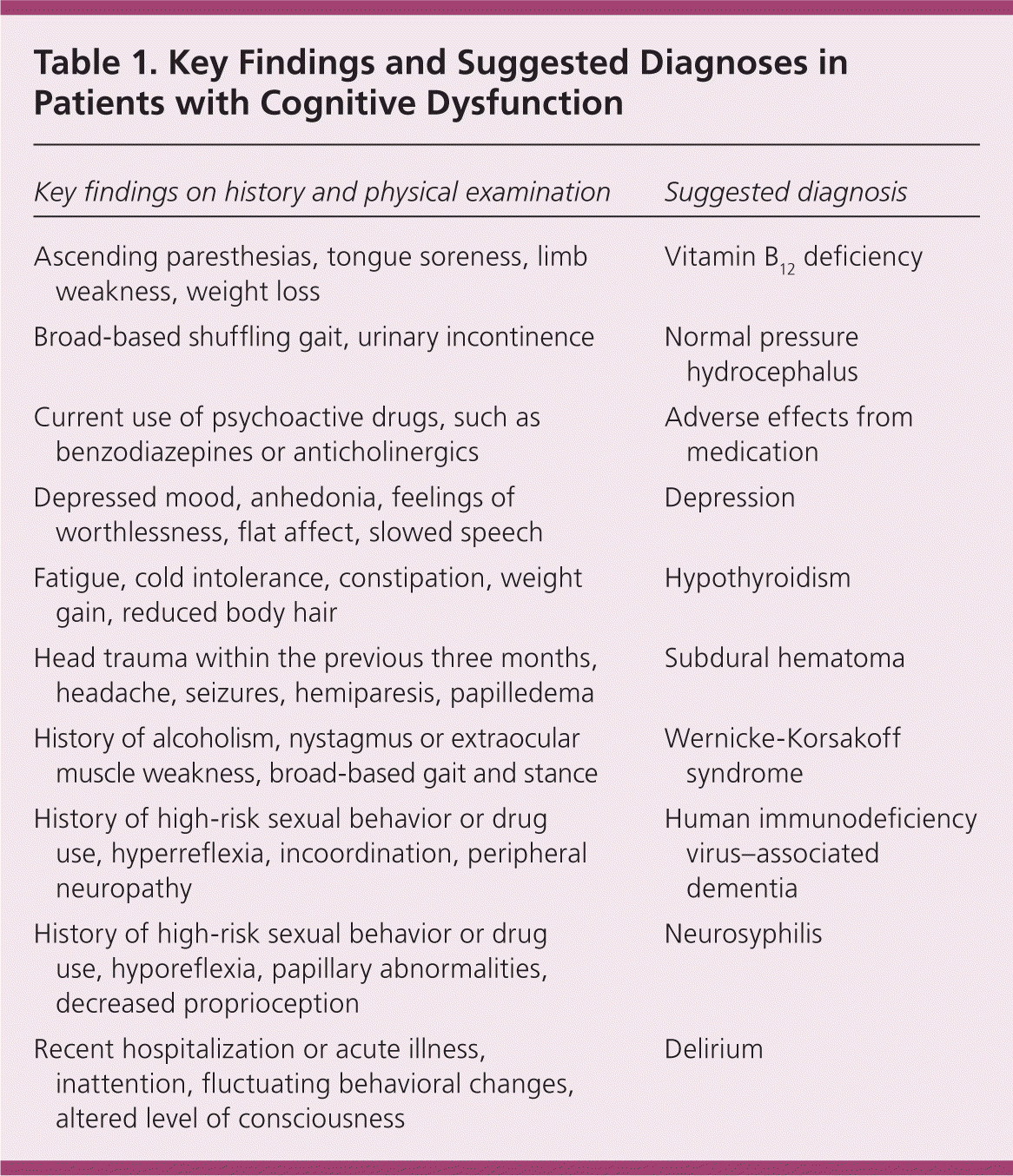
| Key findings on history and physical examination | Suggested diagnosis |
|---|---|
| Ascending paresthesias, tongue soreness, limb weakness, weight loss | Vitamin B12 deficiency |
| Broad-based shuffling gait, urinary incontinence | Normal pressure hydrocephalus |
| Current use of psychoactive drugs, such as benzodiazepines or anticholinergics | Adverse effects from medication |
| Depressed mood, anhedonia, feelings of worthlessness, flat affect, slowed speech | Depression |
| Fatigue, cold intolerance, constipation, weight gain, reduced body hair | Hypothyroidism |
| Head trauma within the previous three months, headache, seizures, hemiparesis, papilledema | Subdural hematoma |
| History of alcoholism, nystagmus or extraocular muscle weakness, broad-based gait and stance | Wernicke-Korsakoff syndrome |
| History of high-risk sexual behavior or drug use, hyperreflexia, incoordination, peripheral neuropathy | Human immunodeficiency virus–associated dementia |
| History of high-risk sexual behavior or drug use, hyporeflexia, papillary abnormalities, decreased proprioception | Neurosyphilis |
| Recent hospitalization or acute illness, inattention, fluctuating behavioral changes, altered level of consciousness | Delirium |
Screening Tests for Cognitive Impairment
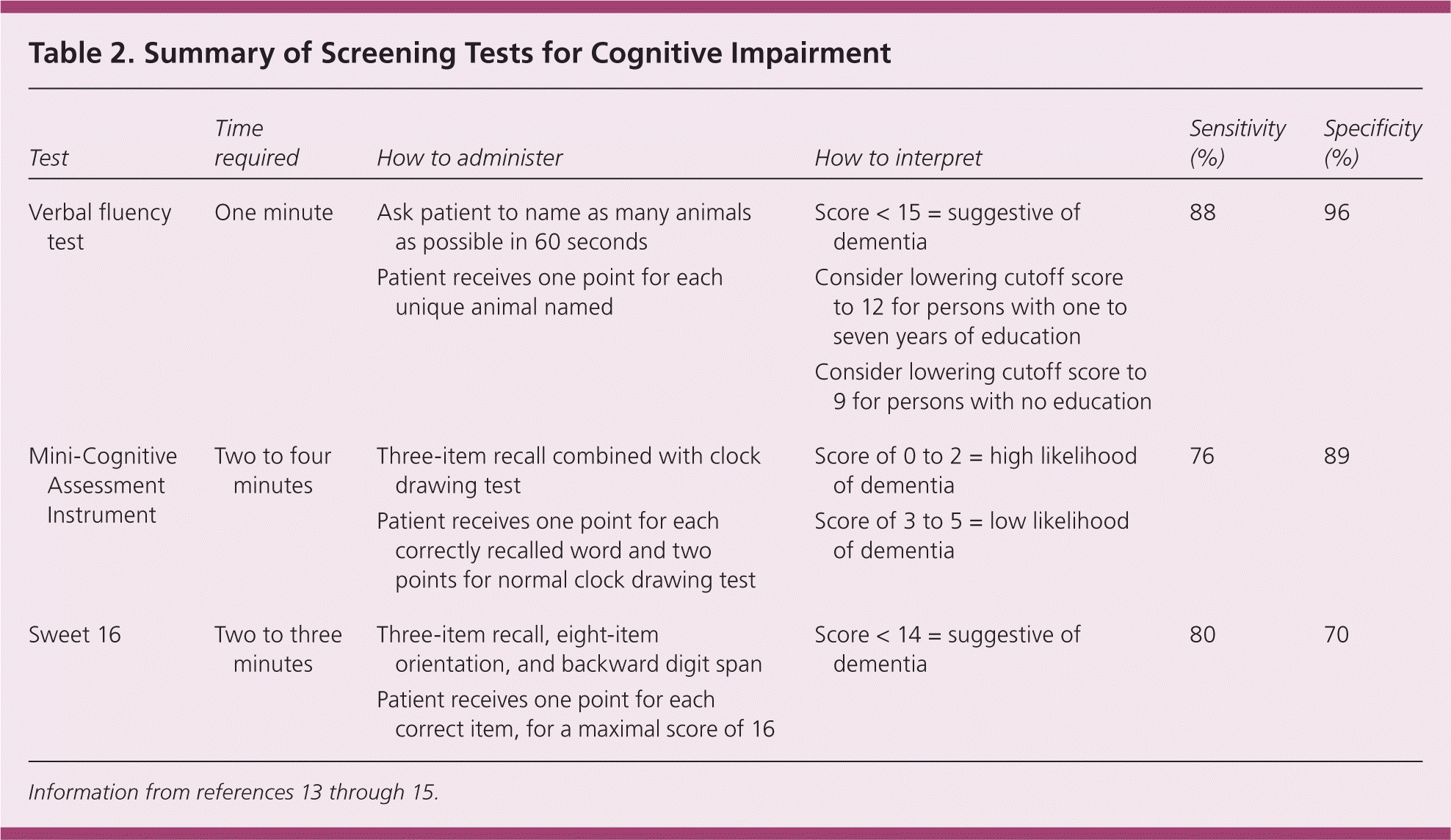
| Test | Time required | How to administer | How to interpret | Sensitivity (%) | Specificity (%) |
|---|---|---|---|---|---|
| Verbal fluency test | One minute |
| Score < 15 = suggestive of dementia | 88 | 96 |
| Consider lowering cutoff score to 12 for persons with one to seven years of education | |||||
| Consider lowering cutoff score to 9 for persons with no education | |||||
| Mini-Cognitive Assessment Instrument | Two to four minutes |
| Score of 0 to 2 = high likelihood of dementia | 76 | 89 |
| Score of 3 to 5 = low likelihood of dementia | |||||
| Sweet 16 | Two to three minutes |
| Score < 14 = suggestive of dementia | 80 | 70 |
VERBAL FLUENCY TEST
Of the two types of verbal fluency tests, category (or semantic) fluency has superior sensitivity and specificity compared with letter (or phonemic) fluency.13 The category fluency test gives participants 60 seconds to name as many items as they can from a specified category, such as animals. The category of animals is the best studied, and should be used routinely in practice. Scores of less than 15 are suggestive of dementia, and scores of 15 or greater are considered normal.13 This cutoff is 88 percent sensitive and 96 percent specific in diagnosing dementia from any cause (positive likelihood ratio = 22.0; negative likelihood ratio = 0.12).13 Lower cutoff scores of 12 for patients with one to seven years of education and 9 for those with no education improve sensitivity, but decrease specificity.16
Verbal fluency testing has the ability to identify patients with mild cognitive impairment, and results can be abnormal up to five years before clinical conversion to dementia.17 Because its results may be abnormal before any other cognitive testing, verbal fluency testing should be included in any complete cognitive assessment. However, it does have an educational bias, and does not give much insight into specific deficits that are obvious in other cognitive tests.
MINI-COG
The Mini-Cognitive Assessment Instrument (Mini-Cog) has high sensitivity and specificity with little to no educational or language bias.18 The Mini-Cog, which can be viewed at https://www.aafp.org/afp/2009/0315/p497.html, combines the clock drawing test (see “Complete Evaluation for Patients Who Screen Positive”) with a three-item recall test. The patient is asked to repeat three unrelated words, then to perform the clock drawing test, and finally to recall the three words.18 The total possible score ranges from 0 to 5, with one point given for each correctly recalled word (only the delayed recall is scored), and two points for a normal clock drawing test. Scores from 0 to 2 are highly suggestive of dementia, whereas scores from 3 to 5 have a low likelihood of dementia.19 The Mini-Cog takes two to four minutes to perform, and has a sensitivity of 76 percent and a specificity of 89 percent in detecting dementia (positive likelihood ratio = 7.0; negative likelihood ratio = 0.27).14
THE SWEET 16
The Sweet 16 combines a three-item recall with eight items of orientation and a backward digit span. Its main advantage over the Mini-Cog is that it can be easily administered to patients who are unable to use a pen. The Sweet 16 takes two to three minutes to perform, and has a sensitivity of 80 percent and a specificity of 70 percent (positive likelihood ratio = 2.7; negative likelihood ratio = 0.28).15
Complete Evaluation for Patients Who Screen Positive
Patients with an abnormal screening evaluation (verbal fluency test, Mini-Cog, or Sweet 16) should have follow-up cognitive testing and further laboratory testing, with or without imaging studies, to rule out secondary causes of dementia. If not completed during the screening evaluation, the verbal fluency and clock drawing tests should be performed at the follow-up evaluation.
MINI-MENTAL STATE EXAMINATION
The Mini-Mental State Examination (MMSE) is a 30-item questionnaire administered by the physician.20 The MMSE, which can be viewed at https://www.aafp.org/afp/2001/0215/p703.html, assesses domains of orientation, attention, concentration, memory, language, and construction abilities. It has good sensitivity (71 to 92 percent) and specificity (56 to 96 percent).21 Lower educational levels can affect patients' MMSE scores, and information on literacy and educational status should be gathered before scoring.22,23 At any given age, average MMSE scores are proportionally higher in persons with higher educational levels.22 The MMSE takes five to 12 minutes, with patients who have cognitive impairments usually taking much longer than those with normal cognition.14 Factors shown to be independent predictors of lower scores include occupational status, living alone, stress, physical strain, and physical inactivity.23
A cognitive test that checks multiple domains is important for evaluating specific deficits, and should be the cornerstone of complete cognitive assessments. There are validated alternatives to the MMSE that test various domains, including the Montreal Cognitive Assessment.24 Patients with an abnormal screening result should complete the MMSE, the Montreal Cognitive Assessment, or a similar test in a follow-up visit.
CLOCK DRAWING TEST
The clock drawing test is the only quick cognitive assessment tool that evaluates organization and planning; therefore, it should be a part of any complete cognitive assessment.25 The patient is given a blank sheet of paper and told to draw the face of a clock with all the numbers, and then set the time to 10 minutes past 11. The clock drawing test has been well studied and validated; however, there is no consensus on how to score it, with more than 15 different validated scoring systems available.26 To avoid the complicated scoring systems, the clock drawing test can be graded as normal (time correctly shown with short hour hand and long minute hand, and numbers in the appropriate location) or abnormal (any other result) when it is used as part of a complete cognitive assessment or as part of the Mini-Cog. Alternatively, the clock drawing test is already incorporated into the Montreal Cognitive Assessment tool with a simple three-point grading system.24
GERIATRIC DEPRESSION SCALE
Dementia and depression share many signs in older persons, including apathy, the inability to concentrate, societal withdrawal, and dramatic changes in mood and affect. The American Academy of Neurology and the American Geriatrics Society (AGS) recommend screening for depression as part of the assessment for dementia.27,28 The Geriatric Depression Scale (GDS-15) is a series of 15 yes/no questions; a score of 5 or greater is considered positive (Figure 129 ).30 The GDS-15 takes approximately three minutes to administer and has a sensitivity of 72 to 93 percent and a specificity of 65 to 78 percent for depresson.30,31
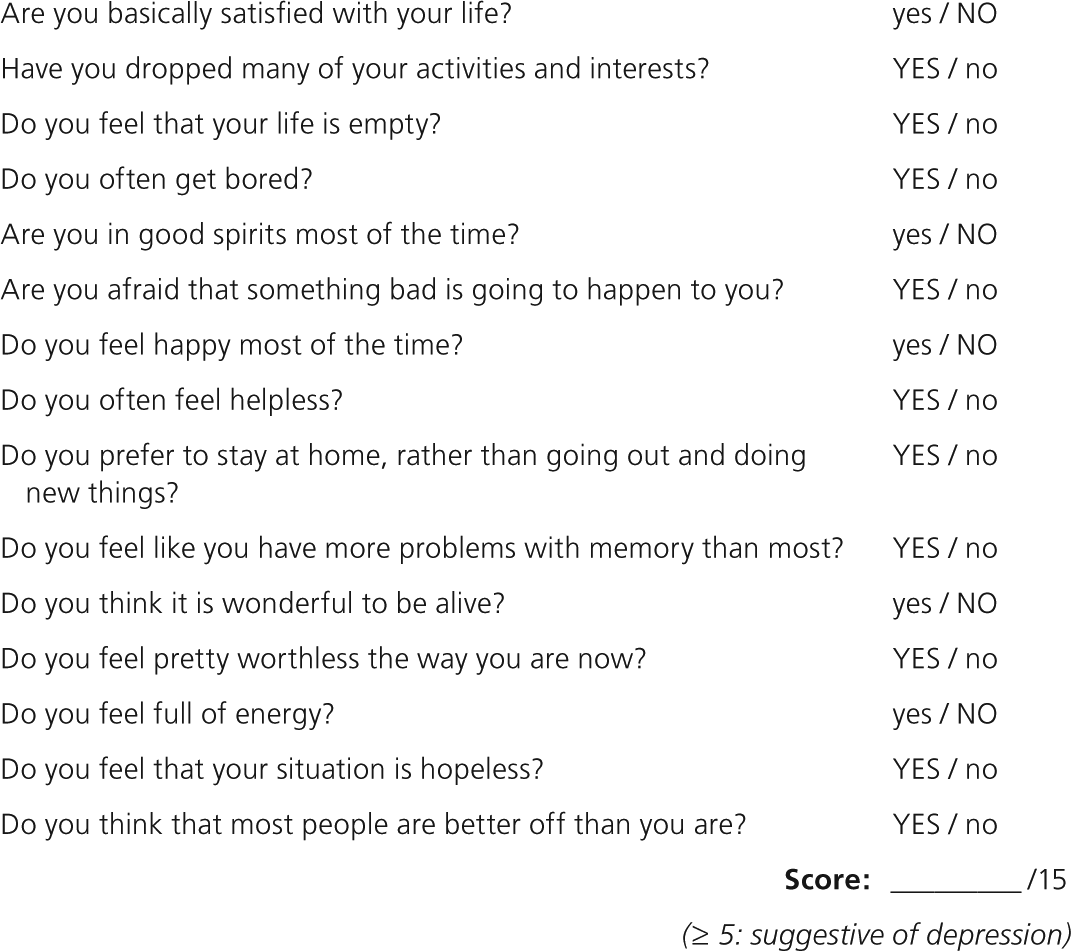
A positive GDS-15 can help differentiate between dementia and pseudodementia. Patients with pseudodementia will demonstrate cognitive impairment from depression instead of a dementia syndrome. These patients seem to exert less effort in cognitive testing and report more perceived cognitive deficits than patients with true dementia.32 Controversy exists over whether the cognitive decline of pseudodementia is reversible with the treatment of depression, with some studies demonstrating that more than one-half of patients eventually progress to dementia.33
LABORATORY AND IMAGING EVALUATION
It is recommended that any assessment for suspected dementia include an initial laboratory evaluation to rule out confounders of memory or reversible causes of memory loss. The American Academy of Neurology recommends testing of vitamin B12 levels and thyroid function for routine initial evaluation. 28 In addition to these tests, the AGS recommends adding a complete blood count and a complete metabolic panel, as well as checking folate levels (Table 327,28 ).27 If the patient has a history of risk factors for sexually transmitted infections, testing for syphilis and human immunodeficiency virus (HIV) infection should be added.27 Other testing such as urinalysis, urine culture, and heavy metal screening should be performed when clinical suspicion is high. Lumbar puncture with cerebrospinal fluid analysis may be indicated if there is suspicion of neurosyphilis, HIV infection, cerebral Lyme disease, or vasculitis.27
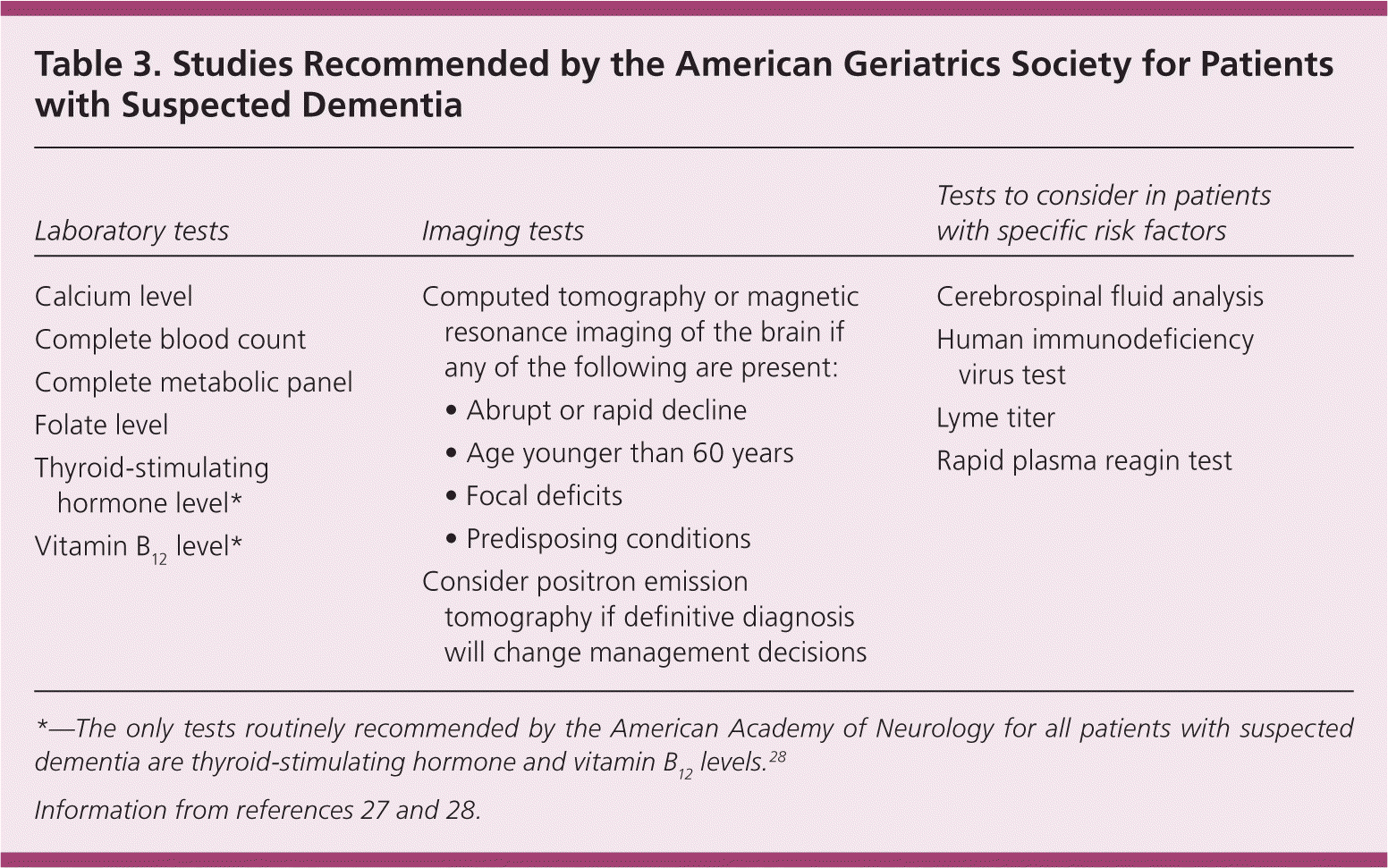
| Laboratory tests | Imaging tests | Tests to consider in patients with specific risk factors |
|---|---|---|
| Calcium level |
|
|
| Complete blood count | ||
| Complete metabolic panel | ||
| Folate level | ||
| Thyroid-stimulating hormone level* | ||
| Vitamin B12 level* |
The yield for neuroimaging is low (approximately 5 percent); however, it may be useful in some symptomatic patients.28 Neuroimaging via computed tomography or magnetic resonance imaging of the brain may detect clinically significant structural lesions that would otherwise be missed. The AGS recommends neuroimaging in patients with any of the following: onset of symptoms before 60 years of age; abrupt onset or rapid cognitive decline (weeks to months); focal neurologic symptoms; or predisposing conditions such as malignancy, HIV disease, or concurrent anticoagulation.27 Neuroimaging should also be considered if vascular disease, normal pressure hydrocephalus, infection, or subdural hematoma is suspected. If imaging studies are indicated, magnetic resonance imaging without contrast media is the preferred study.34
Newer diagnostic methods, such as positron emission tomography (PET) and evaluation of cerebrospinal fluid biomarkers, have been shown to have good diagnostic sensitivity.35,36 One study has shown a significant relationship between levels of cerebrospinal fluid biomarkers, such as beta amyloid and tau protein, and the development of Alzheimer disease and mild cognitive impairment.35 PET can help differentiate among types of dementia, including frontotemporal dementia.37 Another study showed PET with Pittsburgh Compound B protocol to accurately measure the amount of amyloid in the brain and predict Alzheimer disease.36 The implications and benefits of these novel approaches in research settings are straightforward, although their role in clinical medicine is unclear because of issues such as availability, cost, and lack of effective treatment. PET can be considered if differentiation among dementia types would affect management.
Data Sources: A search was completed in Medline via Ovid, the National Guidelines Clearinghouse, the Institute for Clinical Systems Improvement, and the Cochrane Database of Systematic Reviews using the following keywords: dementia, Alzheimer's, verbal fluency, Mini-Cog, clock draw test, Mini-Mental State Exam, cognitive assessment, and geriatric depression scale.
Editor's note: Unfortunately, the Sweet 16 Cognitive Assessment Tool is no longer available. It had been freely available on the Internet, but was taken down after a copyright dispute filed by the new copyright holder of the well-known Mini-Mental State Examination (MMSE). The MMSE, created in 1975 by Dr. Marshall Folstein, had been freely available until 2001, when Psychological Assessment Resources (PAR) started managing the test's copyright license. PAR claimed that the Sweet 16 CAT infringed on the MMSE’s copyright. For more background information, see http://www.amednews.com/article/20120130/profession/301309944/4/.
See additional comment in the comments section below.
