
Am Fam Physician. 2012;85(4):403-404
Guideline source: American College of Obstetricians and Gynecologists
Evidence rating system used? Yes
Literature search described? Yes
Guideline developed by participants without relevant financialties to industry? Not reported
Published source:Obstetrics & Gynecology, July 2011
Available at: http://journals.lww.com/greenjournal/Citation/2011/07000/Practice_Bulletin_No_121_Long_Acting_Reversible.31.aspx (subscription required)
The use of long-acting reversible contraceptives (LARCs) has increased from 1.3 to 5.5 percent over the past decade. There are three LARCs available in the United States: the etonogestrel single-rod implant and two types of intrauterine devices (IUDs; copper T380A and levonorgestrel intrauterine system [Mirena]). Rates of unintended pregnancy are lower and continuation of the contraceptive is higher in women who use LARCs compared with other methods (see accompanying table). LARCs are also less expensive than other methods and do not require ongoing efforts by the patient for long-term effectiveness.
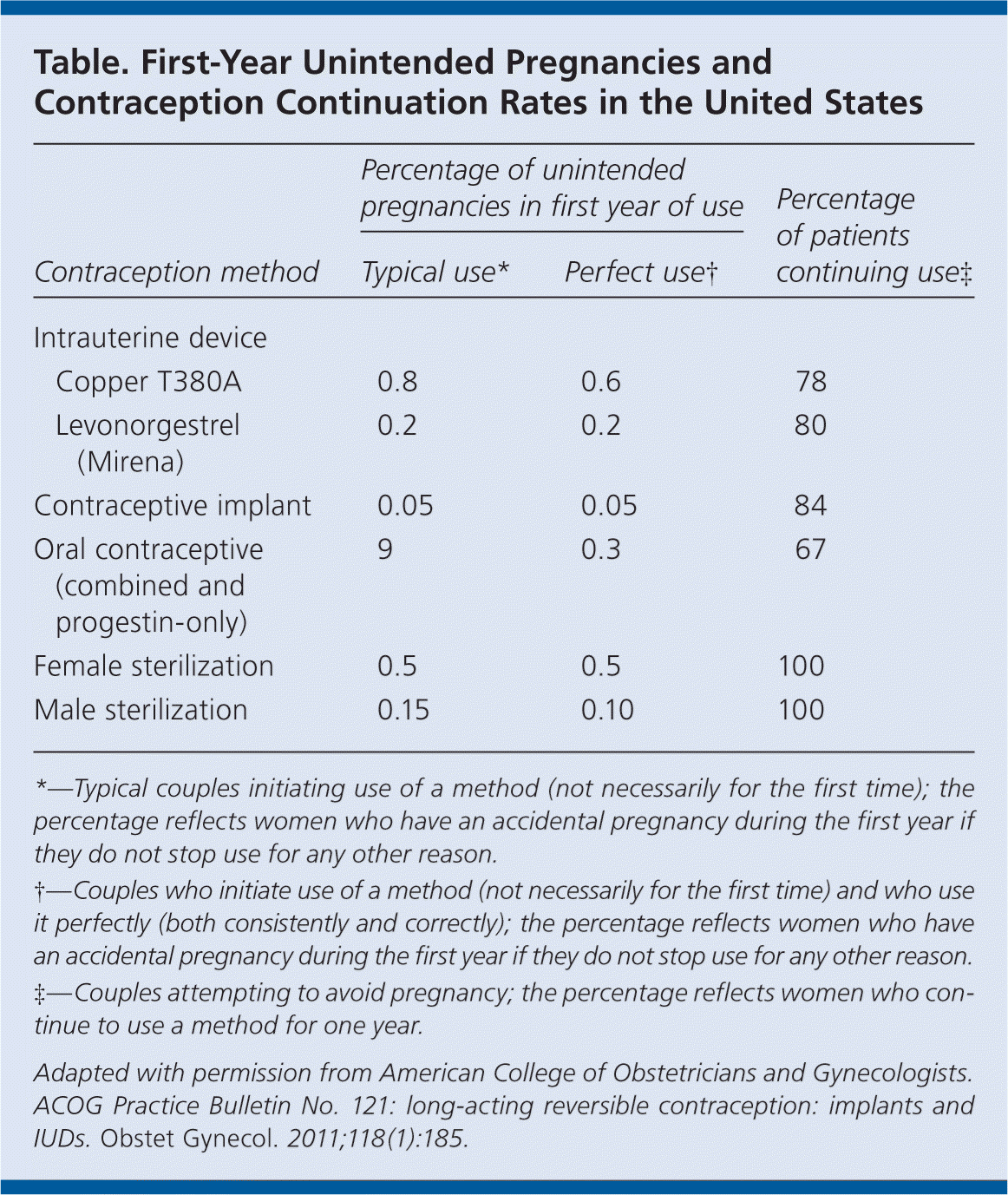
| Percentage of unintended pregnancies in first year of use | Percentage of patients continuing use‡ | |||
|---|---|---|---|---|
| Contraception method | Typical use* | Perfect use † | ||
| Intrauterine device | ||||
| Copper T380A | 0.8 | 0.6 | 78 | |
| Levonorgestrel (Mirena) | 0.2 | 0.2 | 80 | |
| Contraceptive implant | 0.05 | 0.05 | 84 | |
| Oral contraceptive (combined and progestin-only) | 9 | 0.3 | 67 | |
| Female sterilization | 0.5 | 0.5 | 100 | |
| Male sterilization | 0.15 | 0.10 | 100 | |
Encouraging appropriate patients to use LARCs may help lower the rate of unintended pregnancies in the United States, especially in high-risk women. There are few contraindications for the use of LARCs, even in nulliparous women and adolescents. The American College of Obstetricians and Gynecologists has released a practice bulletin to guide physicians on the use of LARCs.
Placement
Although LARCs traditionally have been placed during menses, they may be placed at any time during the menstrual cycle as long as pregnancy can be reasonably excluded. The immediate postpartum period (within 10 minutes of placental separation), including after an abortion, is a favorable time to place LARCs because most of these women are motivated to use contraception, they are known to not be pregnant, and the hospital is a convenient setting for placement. However, LARCs are contraindicated in women with peripartum chorioamnionitis, endometritis, or puerperal sepsis. Although the copper IUD may be preferred because it does not contain hormones, limited evidence has shown that the levonorgestrel intrauterine system and etonogestrel implant do not affect breastfeeding duration or infant growth.
Adverse Effects
Use of LARCs affects menstrual bleeding (e.g., irregular or unpredictable bleeding), and patients should be educated about these effects. New-onset abnormal bleeding should be evaluated as in women who use non-LARCs. There is no evidence that LARCs affect weight, acne, or bone mineral density. The benefits of LARC use outweigh the risks in patients with diabetes mellitus.
Pregnancy
IUDs should be removed in women who become pregnant, if this can be achieved without an invasive procedure. Risks of not removing the device include spontaneous abortion and septic abortion. LARCs do not increase the risk of ectopic pregnancy, and may be used in patients with a history of ectopic pregnancy.
Menopause
It is advisable to wait for one year of amenorrhea before removing a copper IUD. Follicle-stimulating hormone levels should be measured to confirm menopause in those using the levonorgestrel intrauterine system because amenorrhea may be a secondary effect of this method. However, there is no compelling evidence that IUDs must be removed after menopause.
Cervical Procedures
Endometrial biopsy, cervical colposcopy, cervical ablation or excision, and endometrial sampling may be performed with an IUD in place. The IUD string may be tucked in the cervical canal or cut during these procedures.
Actinomyces on Cervical Cytology
Expectant management is appropriate if Actinomyces is detected on cervical cytology in asymptomatic patients with an IUD. This includes education regarding the small risk of actinomycosis.
Emergency Contraception
No randomized controlled trials have been conducted to compare IUDs with medical regimens for emergency contraception, and the levonorgestrel intrauterine system has not been studied for this use. The copper IUD is the most effective method of emergency contraception when inserted up to five days after unprotected sex.
STI Screening
Routine screening for sexually transmitted infections (STIs) is recommended before IUD placement in high-risk women, but not in low-risk women. Screening may be performed on the same day as the IUD placement. Although the risk of pelvic inflammatory disease is higher in women with an STI at the time of IUD placement, this risk is low. Women with mucopurulent discharge or with known chlamydia or gonorrhea cervicitis should be treated before an IUD is placed.
Antibiotic Prophylaxis
Routine use of antibiotics to prevent pelvic infections is not recommended before IUD placement. Randomized controlled trials show that antibiotic prophylaxis does not reduce the risk of pelvic infection or the need to remove the IUD in the first three months after placement.
