Am Fam Physician. 2012;85(8):791-796
A more recent article on pelvic inflammatory disease is available.
Patient information: See related handout on pelvic inflammatory disease, written by the author of this article.
Author disclosure: No relevant financial affiliations to disclose.
Pelvic inflammatory disease is a polymicrobial infection of the upper genital tract. It primarily affects young, sexually active women. The diagnosis is made clinically; no single test or study is sensitive or specific enough for a definitive diagnosis. Pelvic inflammatory disease should be suspected in at risk patients who present with pelvic or lower abdominal pain with no identified etiology, and who have cervical motion, uterine, or adnexal tenderness. Chlamydia trachomatis and Neisseria gonorrhoeae are the most commonly implicated microorganisms; however, other microorganisms may be involved. The spectrum of disease ranges from asymptomatic to life-threatening tubo-ovarian abscess. Patients should be treated empirically, even if they present with few symptoms. Most women can be treated successfully as outpatients with a single dose of a parenteral cephalosporin plus oral doxycycline, with or without oral metronidazole. Delay in treatment may lead to major sequelae, including chronic pelvic pain, ectopic pregnancy, and infertility. Hospitalization and parenteral treatment are recommended if the patient is pregnant, has human immunodeficiency virus infection, does not respond to oral medication, or is severely ill. Strategies for preventing pelvic inflammatory disease include routine screening for chlamydia and patient education.
Pelvic inflammatory disease (PID) is a polymicrobial infection of the upper genital tract that primarily affects young, sexually active women. There are 750,000 cases of PID each year in the United States, mainly in women 15 to 29 years of age.1 This number has remained constant since the early 1990s, after decreasing in the previous decades. Most women are treated in the outpatient setting. The number of hospitalizations has steadily decreased over the past decade.1 The cost of PID is approximately $2,000 per patient, which equals about $1.5 billion annually.2 It is estimated that 80 to 90 percent of women with a genital chlamydial infection and 10 percent with gonorrheal infection are asymptomatic. Approximately 10 to 20 percent of women with chlamydial or gonorrheal infections may develop PID if not treated. Women with PID have a 20 percent chance of developing infertility from tubal scarring, a 9 percent chance of having an ectopic pregnancy, and an 18 percent chance of developing chronic pelvic pain.3,4 In 2010, the Centers for Disease Control and Prevention updated its PID guidelines, which are the basis of this review.5
| Clinical recommendation | Evidence rating | References |
|---|---|---|
| No single clinical finding or laboratory test is sensitive or specific enough to definitively diagnose PID. | C | 7 |
| Empiric antibiotic treatment should be initiated at the time of presentation in patients with symptoms suspicious for PID, even if the diagnosis has not been confirmed. | B | 15 |
| Women with mild to moderate PID may receive outpatient oral medical treatment without increased risk of long-term sequelae. | B | 18, 19 |
| Unless there is proven sensitivity, fluoroquinolones should not be used in women with PID because of widespread resistance in Neisseria gonorrhoeae; a parenteral cephalosporin is recommended instead. | C | 20 |
| Screening for lower genital tract chlamydial infection in younger and high-risk populations is recommended to reduce the incidence of PID. Asymptomatic disease should be treated. | A | 26, 27 |
| The frequency and cost-effectiveness of screening for asymptomatic lower genital tract chlamydial infection are not clear. | C | 28, 29 |
Pathophysiology
The microorganisms that are implicated in PID are thought to spread in three ways:
Intra-abdominally, traveling from the cervix to the endometrium, through the salpinx, and into the peritoneal cavity (causing endometritis, salpingitis, tubo-ovarian abscess, or pelvic peritonitis)
Through the lymphatic systems, such as infection of the parametrium from an intrauterine device (IUD)
Through hematogenous routes, such as with tuberculosis, although this is rare.6
Diagnosis
The diagnosis of PID is based primarily on clinical evaluation. Because of the potential for significant consequences if treatment is delayed, physicians should treat on the basis of clinical judgment without waiting for confirmation from laboratory or imaging tests. Most importantly, physicians must consider PID in the differential diagnosis in women 15 to 44 years of age who present with lower abdominal or pelvic pain and cervical motion or pelvic tenderness, even if these symptoms are mild. However, there is no single symptom, physical finding, or laboratory test that is sensitive or specific enough to definitively diagnose PID7; clinical diagnosis alone is 87 percent sensitive and 50 percent specific.8 When compared with laparoscopy, clinical diagnosis of PID in symptomatic patients has a positive predictive value of 65 to 90 percent.5 This depends on the risk factors within the population being evaluated.
HISTORY AND PHYSICAL EXAMINATION
The history should focus on high-risk behaviors and symptoms. Risk factors for PID include age younger than 25 years; young age at first sexual encounter (younger than 15 years); use of nonbarrier contraception, especially IUD or oral contraceptives; new, multiple, or symptomatic sex partners; a history of PID or sexually transmitted infection; or recent IUD insertion.9 Black women may be at higher risk of PID, although there are inconsistencies among physicians in their criteria for diagnosing PID and biases in reporting.1,10 Vaginal douching also may be a risk factor.11
Typically, women will present with some degree of lower abdominal or pelvic pain, although it may be mild. Other symptoms may include a new or abnormal vaginal discharge, fever or chills, cramping, dyspareunia, dysuria, and abnormal or postcoital bleeding. Some women also may have low back pain, nausea, and vomiting.6 It is less common for women to have no symptoms or atypical symptoms, such as right upper quadrant pain from perihepatitis (i.e., Fitz-Hugh–Curtis syndrome). At-risk women who present with pelvic or lower abdominal pain and have no other identified etiology for their pain should be presumed to have PID if they have cervical motion, uterine, or adnexal tenderness. Additional diagnostic criteria are outlined in Table 1.5 The differential diagnosis also may include gastrointestinal conditions (e.g., acute appendicitis, inflammatory bowel disease); genitourinary conditions (e.g., urinary tract infection/pyelonephritis, nephrolithiasis); obstetric/gynecologic conditions (e.g., ovarian tumor/cyst/torsion, ectopic pregnancy); or functional pelvic pain.

| One or more of the following minimum criteria must be present on pelvic examination to diagnose PID: | |
| Cervical motion tenderness | |
| Uterine tenderness | |
| Adnexal tenderness | |
| The following criteria can improve the specificity of the diagnosis: | |
| Oral temperature > 101°F (> 38.3°C) | |
| Abnormal cervical or vaginal mucopurulent discharge | |
| Presence of abundant numbers of white blood cells on saline microscopy of vaginal fluid | |
| Elevated erythrocyte sedimentation rate | |
| Elevated C-reactive protein level | |
| Laboratory documentation of cervical infection with gonorrhea or chlamydia | |
| The following test results are the most specific criteria for diagnosing PID: | |
| Endometrial biopsy with histopathologic evidence of endometritis | |
| Transvaginal sonography or magnetic resonance imaging techniques showing thickened, fluid-filled tubes with or without free pelvic fluid or tubo-ovarian complex, or Doppler studies suggesting pelvic infection (e.g., tubal hyperemia) | |
| Laparoscopic abnormalities consistent with PID | |
DIAGNOSTIC STUDIES
Nucleic acid amplification tests (e.g., strand displacement amplification, ligase chain reaction, or polymerase chain reaction testing) for Chlamydia trachomatis and Neisseria gonorrhoeae are sensitive (90 to 98 percent and 88.9 to 95.2 percent, respectively), specific (98 to 100 percent and 99.1 to 100 percent, respectively), and cost-effective for documenting the presence of these organisms.12 These tests can be used for vaginal or endocervical specimens collected by the physician, vaginal specimens self-collected by the patient, or urine samples.13 Sensitivity to antimicrobial agents can be tested only on cultures.
Other diagnostic studies are rarely indicated unless there is no response to treatment. In addition to increasing the cost to the patient, these tests create additional risks from the procedure itself or from anesthesia. Although they are not needed routinely, the tests that are most specific for diagnosing PID include endometrial biopsy with histopathologic evidence of endometritis (74 percent sensitive; 84 percent specific); transvaginal sonography (30 percent sensitive; 76 percent specific), particularly with Doppler flow assessment; magnetic resonance imaging techniques showing thickened, fluid-filled tubes; or laparoscopic abnormalities consistent with salpingitis or peritonitis (81 percent sensitive; 100 percent specific).8 Patients who do not have a clear diagnosis despite radiologic studies or who are not responding to therapy may benefit from laparoscopy. If no evidence of PID is identified after laparoscopy, endometrial biopsy may be required to evaluate for endometritis. Computed tomography has been used to diagnose PID; however, this is an insensitive and expensive test that is inaccessible to many high-risk patients, and generates extra radiation exposure.
Treatment and Management
According to consensus data, the treatment of PID must be empiric because a definitive diagnosis is rarely known or confirmed at the time of presentation.15 Empiric treatment may result in adverse effects from the antibiotics, including allergic reactions, gastrointestinal symptoms, or drug resistance; however, the benefits are thought to outweigh the risks.5 Because these infections are polymicrobial, broad-spectrum antimicrobial agents are recommended to cover the most likely pathogens. The antibiotics employed should be effective against C. trachomatis and N. gonorrhoeae even if the tests are negative, because women may have upper genital tract disease without cervical cultures that are positive for these organisms.5
There is controversy over the need for treatment that is effective against anaerobic bacteria in women with PID. Anaerobic microorganisms found in patients with PID may include anaerobic vaginal and cervical flora, such as bacterial vaginosis–related organisms (e.g., Gardnerella vaginalis).16 Guidelines recommend targeting these organisms in the absence of definitive study outcomes. Mycoplasma genitalium infection has been associated with treatment failure in some women, and current treatment guidelines do not target this organism. More studies are needed to determine if treatments targeting this organism should be routinely recommended.17
Data comparing the effectiveness of different treatment regimens are limited; guidelines are updated to reflect resistance patterns and additional organisms that are implicated in these infections. Initially, physicians should determine whether the patient requires inpatient or outpatient management.18 Criteria for hospitalization are listed in Table 2.5 Randomized clinical trials have demonstrated effectiveness of parenteral and oral antimicrobial agents in patients with mild or moderate PID.18 The oral and parenteral treatment regimen options are listed in Tables 3 and 4.5 The first option for oral treatment includes a onetime 250-mg intramuscular dose of ceftriaxone (Rocephin) plus 100 mg of doxycycline orally twice per day for 14 days. Women with mild to moderate PID may receive outpatient oral medical treatment without increased risk of long-term sequelae.18,19 The patient's age does not affect the response to treatment, whether inpatient or outpatient.
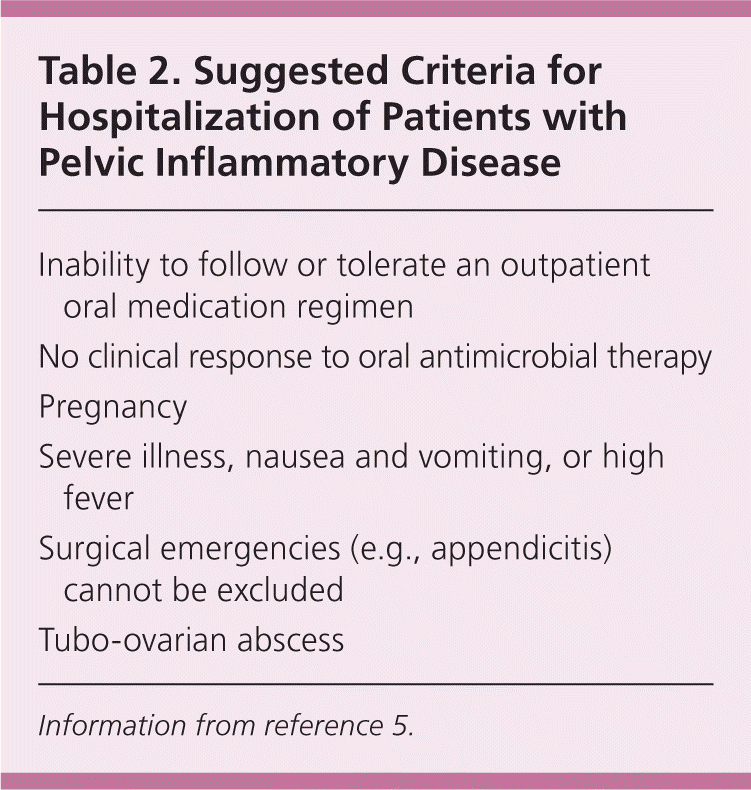
| Inability to follow or tolerate an outpatient oral medication regimen |
| No clinical response to oral antimicrobial therapy |
| Pregnancy |
| Severe illness, nausea and vomiting, or high fever |
| Surgical emergencies (e.g., appendicitis) cannot be excluded |
| Tubo-ovarian abscess |

| Drug | Dosage | |
|---|---|---|
| Option 1 | ||
| Ceftriaxone (Rocephin) | 250 mg IM in a single dose | |
| plus | ||
| Doxycycline | 100 mg orally twice per day for 14 days | |
| with or without | ||
| Metronidazole (Flagyl) | 500 mg orally twice per day for 14 days | |
| Option 2 | ||
| Cefoxitin | 2 g IM in a single dose administered concurrently with probenecid (1 g orally) | |
| plus | ||
| Doxycycline | 100 mg orally twice per day for 14 days | |
| with or without | ||
| Metronidazole | 500 mg orally twice per day for 14 days | |
| Option 3 | ||
| Other parenteral third-generation cephalosporin (e.g., ceftizoxime [Cefizox], cefotaxime [Claforan]) | ||
| plus | ||
| Doxycycline | 100 mg orally twice per day for 14 days | |
| with or without | ||
| Metronidazole | 500 mg orally twice per day for 14 days | |

| Drug | Dosage | |
|---|---|---|
| Regimen A | ||
| Cefotetan (Cefotan) | 2 g IV every 12 hours | |
| or | ||
| Cefoxitin | 2 g IV every six hours | |
| plus | ||
| Doxycycline | 100 mg orally or IV every 12 hours | |
| Regimen B | ||
| Clindamycin | 900 mg IV every eight hours | |
| plus | ||
| Gentamicin | Loading dose IV or IM (2 mg per kg), followed by a maintenance dose (1.5 mg per kg) every eight hours; a single daily dose (3 to 5 mg per kg) can be substituted | |
| Alternative regimen | ||
| Ampicillin/sulbactam (Unasyn) | 3 g IV every six hours | |
| plus | ||
| Doxycycline | 100 mg orally or IV every 12 hours | |
If parenteral therapy is required, the patient should be transitioned to oral treatment 24 to 48 hours after clinical improvement. Women with tubo-ovarian abscesses should have at least 24 hours of inpatient treatment, and may require additional treatment, such as surgery.18 There is widespread emergence of N. gonorrhoeae resistance to fluoroquinolones, and these agents are no longer recommended unless there is a positive culture with confirmed sensitivity. Otherwise, a parenteral cephalosporin is suggested.20
Follow-up is important to ensure that the patient is responding to outpatient treatment. Clinical symptoms should improve within 72 hours of treatment, and if not, further evaluation is advised. Some patients may require additional testing to rule out other diagnoses, such as a tubo-ovarian abscess, and assessment is needed for additional antimicrobial therapy, parenteral antimicrobials, and hospitalization.
Male partners of women with PID should be evaluated and treated if they have had sexual contact within 60 days of a diagnosis of PID.5 Men are often asymptomatic even when their partners are positive for chlamydia or gonorrhea. To decrease the chance of recurrence, women and their partners should abstain from sexual intercourse until they have completed the course of treatment.
Women with PID should be counseled about the prevention of sexually transmitted infections and PID because there is a high risk of reinfection even when partners have been treated. Repeat testing for women with chlamydia or gonorrhea is suggested three to six months after treatment.21 Testing for human immunodeficiency virus (HIV) infection and syphilis should be performed to rule out coexisting infections.
SPECIAL POPULATIONS
Women with HIV infection may be less likely to test positive for gonorrhea or chlamydia, but are more likely to have an infection from Mycoplasma or Streptococcus species.22 Oral and parenteral treatments are equally effective in women with and without HIV infection, unless there is a tubo-ovarian abscess, which, though rare, occurs more often in those with HIV.23
PID is uncommon during pregnancy, although if it occurs, it is usually within the first 12 weeks before the mucous plug can act as an adequate barrier. Pregnant women with suspected PID should be hospitalized and given parenteral antibiotics. PID during pregnancy increases the risk of preterm delivery and increases maternal morbidity.24
Women with IUDs have an increased risk of PID only within the first three weeks after insertion of the IUD. There is no evidence that suggests removal of the IUD is necessary in patients with acute PID; however, close follow-up is recommended. Data indicate no difference in outcomes of PID in women with copper IUDs versus the levonorgestrel-releasing intrauterine system (Mirena). There are insufficient data to suggest that antibiotics should be given to patients at the time of IUD insertion to decrease the risk of developing infection.25
Screening and Prevention
Screening for chlamydia and gonorrhea in young women has been shown to decrease the incidence of PID in high-risk populations. The U.S. Preventive Services Task Force recommends screening for chlamydia in all sexually active women younger than 25 years and in those 25 years and older at increased risk.26,27 There is evidence that screening more than once per year may be more effective than annual screening in high-risk groups. One study showed that 79 percent of the women who developed PID over a 12-month period tested negative on their annual chlamydia screening.28,29 Women should be screened each time they have a new sex partner. However, data are unclear on whether it is cost-effective to screen women who are asymptomatic for lower tract chlamydial infections.28,29
There is some evidence that adolescents respond to brief behavioral intervention at the time PID is diagnosed to increase the chances that their partners will receive treatment, thus decreasing recurrence.30 There is also evidence that counseling can help decrease the risk of PID in at-risk populations.31 Counseling about condom use can decrease the risk of PID.32 Greater awareness about the importance of screening and adequate education on PID prevention are needed for high-risk populations.
Data Sources: A PubMed search was completed using the following key terms: PID, pelvic inflammatory disease, sexually transmitted infections or sexually transmitted diseases AND chlamydia and gonorrhea. The search included meta-analyses, randomized controlled trials, clinical trials, and reviews. Additional searches included the Agency for Healthcare Research and Quality evidence reports, U.S. Preventive Services Task Force, Clinical Evidence, the Cochrane database, Database of Abstracts of Reviews of Effects, the Institute for Clinical Systems Improvement, the National Guideline Clearinghouse, and UpToDate. Initial search date was March 30, 2011, and repeated June 15, 2011. A repeat search in PubMed, UpToDate, and the Cochrane database was performed October 10, 2011.