
Am Fam Physician. 2012;86(8):768-770
Author disclosure: No relevant financial affiliations to disclose.
Rivaroxaban (Xarelto) is a once-daily, orally administered anticoagulant approved by the U.S. Food and Drug Administration (FDA) to reduce the risk of stroke and systemic embolism in patients with nonvalvular atrial fibrillation.1 It is also labeled for the prevention of deep venous thrombosis (DVT) and pulmonary embolism after total knee or hip replacement surgery. Targeting only factor Xa in the coagulation cascade, rivaroxaban has a mechanism of action different from that of warfarin (Coumadin), which inhibits all vitamin K–dependent clotting factors. Therefore, it does not require international normalized ratio (INR) monitoring.
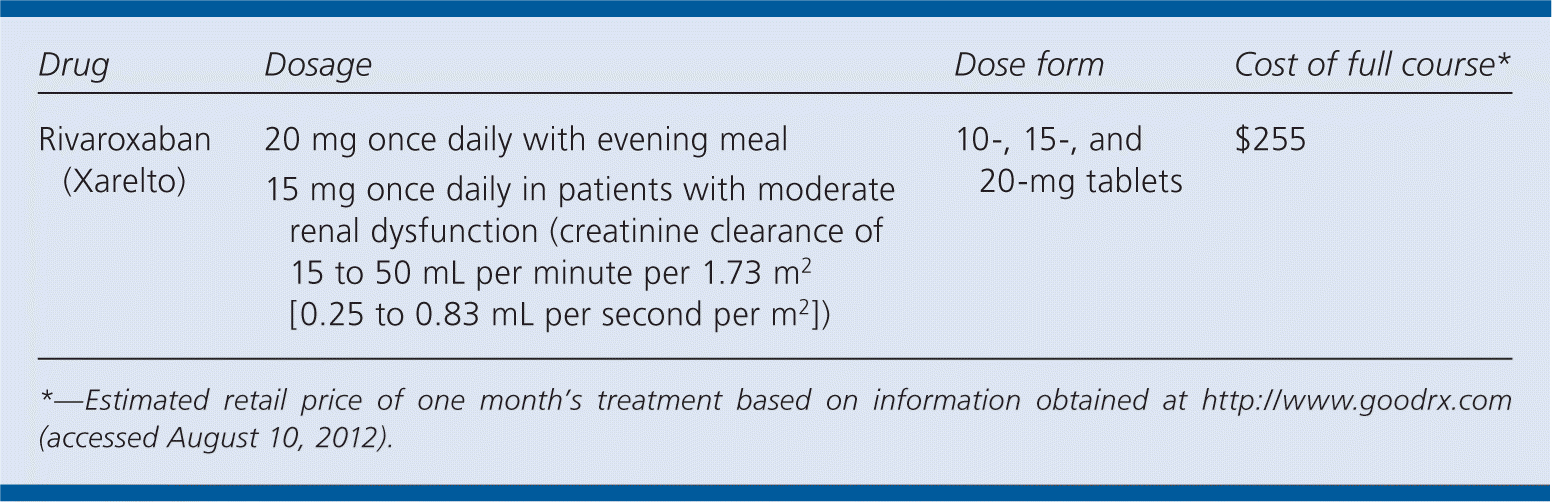
| Drug | Dosage | Dose form | Cost of full course* |
|---|---|---|---|
| Rivaroxaban (Xarelto) | 20 mg once daily with evening meal | 10-, 15-, and 20-mg tablets | $255 |
| 15 mg once daily in patients with moderate renal dysfunction (creatinine clearance of 15 to 50 mL per minute per 1.73 m2 [0.25 to 0.83 mL per second per m2]) |
SAFETY
As with all anticoagulants, rivaroxaban increases the risk of major bleeding events. Rates do not differ significantly between patients who take rivaroxaban and those who take warfarin for all bleeding events (between 14 and 15 percent per year) or for major bleeding (about 3.5 percent per year).2 Bleeding rates are also similar for patients receiving postoperative injectable enoxaparin (Lovenox) and rivaroxaban for all bleeding (about 5 to 6 percent during the two-to five-week postoperative study period) and for major bleeding (0.1 to 0.6 percent during the same period).3,4 Unlike that of warfarin, the anticoagulative effect of rivaroxaban cannot be rapidly reversed. Potential drug interactions should be checked before prescribing rivaroxaban, because its anticoagulant activity can be affected by many medications. Combining rivaroxaban with clopidogrel (Plavix), aspirin, or nonsteroidal anti-inflammatory drugs should be avoided. Rivaroxaban has not been studied in children, pregnant women, or nursing mothers, and is an FDA pregnancy category C drug.1
TOLERABILITY
Rivaroxaban is well tolerated. Patient discontinuation of the drug in clinical investigation was due primarily to bleeding events. Adverse events other than bleeding, such as peripheral edema, dizziness, and heart failure, occurred at similar rates for rivaroxaban and warfarin.2
EFFECTIVENESS
Rivaroxaban has been compared with warfarin for prevention of stroke and systemic embolism in patients with nonvalvular atrial fibrillation. The single study used for marketing approval, an industry-funded randomized controlled trial, included 14,264 high-risk patients2 with an annual stroke risk of 6 to 18 percent based on a CHADS2 score of 2 to 6 (scoring one point each for congestive heart failure, hypertension, age 75 years or older, or diabetes mellitus, and scoring two points for prior stroke).5 Rivaroxaban was as effective as warfarin at preventing stroke and systemic embolism, and reduced annual stroke rates to 2.1 percent, compared with 2.4 percent for warfarin (P = not significant).2 No trials have compared rivaroxaban with dabigatran (Pradaxa), a direct thrombin inhibitor, or with fondaparinux (Arixtra), an injectable factor Xa inhibitor. Rivaroxaban has not been studied for any other type of nonsurgical stroke prevention, and has not been evaluated for safety and effectiveness in patients with intermittent (paroxysmal) atrial fibrillation, cardiac valve replacement, postmyocardial infarction, transient ischemic attack, or other risk factors for stroke.
Rivaroxaban has also proved effective for DVT prophylaxis following total knee or hip arthroplasty. The industry-supported RECORD (Regulation of Coagulation in Orthopedic Surgery to Prevent Deep Venous Thrombosis and Pulmonary Embolism) trials compared rivaroxaban and enoxaparin, with rivaroxaban administered at 10 mg once daily (rather than the standard U.S. regimen of 20 mg once daily), and with enoxaparin administered at the European dosing regimen of 40 mg once daily (rather than the standard U.S. regimen of 30 mg twice daily).3,4 The trials reported a lower one-month postoperative symptomatic thromboembolism rate with rivaroxaban than with enoxaparin for patients who had knee surgery,3 and similar rates for patients who had hip surgery.4 Rivaroxaban has not been studied for any other types of DVT prophylaxis.
PRICE
At a monthly cost of approximately $255 for a 20-mg daily dosage, rivaroxaban is significantly more expensive than warfarin (approximately $6 for a 30-day supply of 1-, 2-, 2.5-, or 5-mg tablets; and between $6 and $7 for a 30-day supply of 7.5- or 10-mg tablets), and roughly the same price as dabigatran (approximately $255 for a one-month supply of 150 mg twice daily).6 The price gap over warfarin may be narrowed by accounting for the elimination of laboratory INR monitoring and corresponding office visits.
SIMPLICITY
Rivaroxaban is an orally administered tablet taken once daily. The dosing is fixed (not based on weight) and requires no INR monitoring. The dosage should be reduced to 15 mg once daily in patients with moderate renal dysfunction (creatinine clearance of 15 to 50 mL per minute per 1.73 m2 [0.25 to 0.83 mL per second per m2]) and should not be used in patients with renal failure.
Bottom Line
In patients at high risk of stroke because of nonvalvular atrial fibrillation, rivaroxaban is as safe and effective as warfarin. However, it should not be used in patients at risk of stroke for reasons other than nonvalvular atrial fibrillation until research shows its relative safety and effectiveness. Serious bleeding cannot be quickly reversed. The key advantages of rivaroxaban are simplicity of use (fixed-dose, once-daily administration), absence of food interactions, and elimination of cumbersome INR monitoring and associated office visits; however, it is many times more expensive than warfarin, and its cost-effectiveness has yet to be established.
