
Am Fam Physician. 2013;88(8):524-530
Related letter: Increase in Reported Malaria Cases Prompts Clarification Regarding Diagnosis and Treatment
Author disclosure: No relevant financial affiliations.
Overall, 3% to 19% of travelers to the developing world will return to the United States with fever or will develop fever within weeks of their return. When evaluating the returning traveler with fever, it is important to know which pretravel immunizations the patient received; which medications he or she took during travel; the likely pathogen exposures during travel; and the incubation interval between travel and onset of fever. A physical examination that includes a search for focal findings may narrow the list of possible infections. Fever compatible with a common illness that occurs in the United States (e.g., mononucleosis) should always be considered. If the patient has fever without a focus and a tropical infection is suspected, malaria, dengue fever, and typhoid fever are common causes. These infections may appear clinically similar, with symptoms of fever, headache, muscle pain, joint pain, and malaise, and decreased white blood cell and platelet counts. Malaria can usually be diagnosed with a thin blood smear. Dengue fever is a clinical diagnosis. Serologic testing for dengue virus immunoglobulin M and G and virus detection tests can be performed to confirm the diagnosis, but are not immediately available. Typhoid fever can usually be diagnosed with a blood, urine, or stool culture.
Overall, 3% to 19% of travelers to the developing world will have a fever on their return to the United States, or will develop fever within weeks of their return. These travelers may present to family physicians who will face the challenge of diagnosing the cause of fever. With a history, physical examination, and laboratory tests, the etiology of the fever can be established for many of these patients.1–4 Fever compatible with a common illness that occurs in the United States (e.g., mononucleosis) should always be considered. The most common diseases in each geographic region are listed in Table 1.5 The GeoSentinel Surveillance Network has 31 clinical sites on six continents that collect data on diseases of returned travelers.1 From 1997 to 2006, it identified 24,920 travelers who had acquired travel-related diseases, 28% of whom had fever (Table 2).1
| Clinical recommendation | Evidence rating | References |
|---|---|---|
| Malaria should be suspected in patients with spiking fevers, leukopenia, thrombocytopenia, and increased bands who have visited a malaria-endemic area and did not take prophylaxis. | C | 11 |
| Persons traveling outside of the United States should use repellents that contain 30% to 50% diethyltoluamide (DEET), because they provide sufficient protection against mosquito bites. | B | 19 |
| When traveling outside the United States, it is usually safe to consume bottled water; hot cooked food; dry food; and fruits and vegetables that can be peeled. Tap water; ice; fresh juices and salads; unpasteurized dairy products; uncooked sauces and toppings; open buffets; and food sold by street vendors should be avoided. | C | 4 |
| Typhoid fever should be suspected in patients with spiking fever and leukopenia who have visited a typhoid-endemic area and who have not received a typhoid fever vaccination. | C | 21 |
| Dengue fever should be suspected in patients with spiking fevers and leukopenia who have visited a dengue-endemic area. | C | 23–25 |
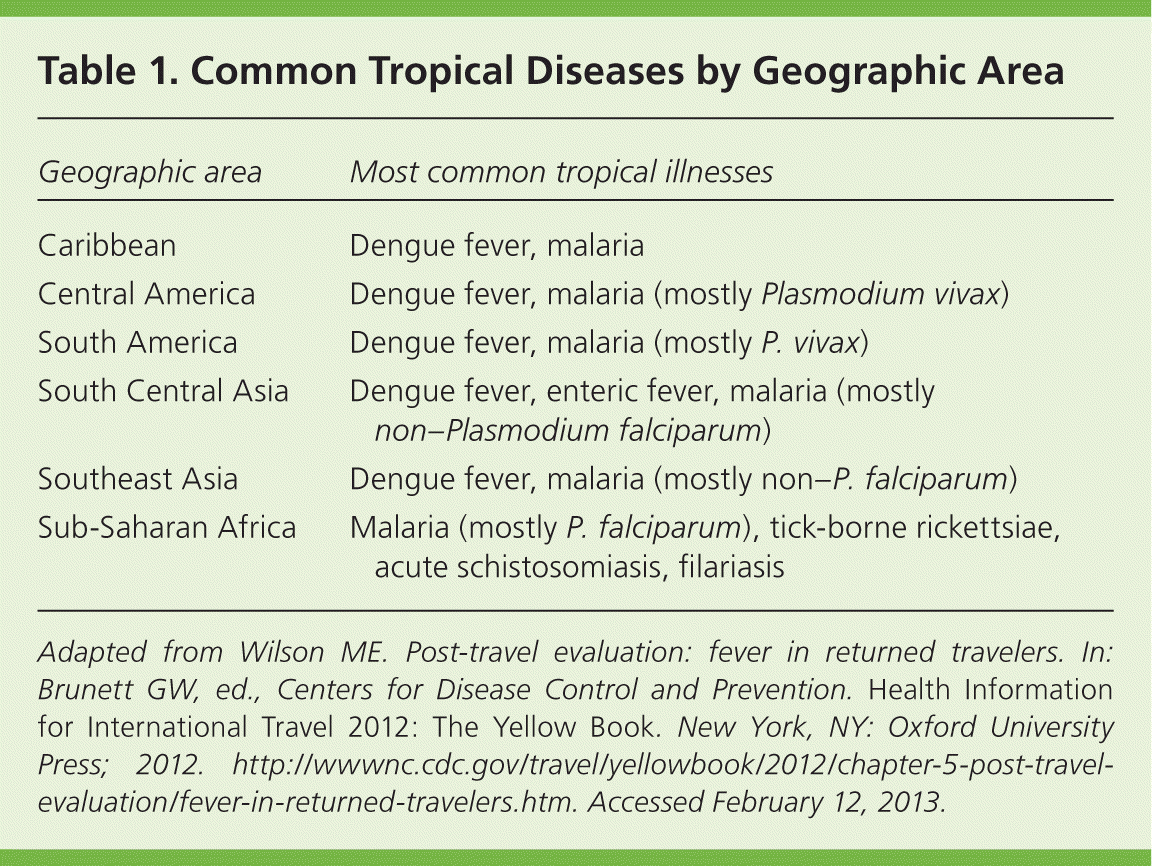
| Geographic area | Most common tropical illnesses |
|---|---|
| Caribbean | Dengue fever, malaria |
| Central America | Dengue fever, malaria (mostly Plasmodium vivax) |
| South America | Dengue fever, malaria (mostly P. vivax) |
| South Central Asia | Dengue fever, enteric fever, malaria (mostly non–Plasmodium falciparum) |
| Southeast Asia | Dengue fever, malaria (mostly non–P. falciparum) |
| Sub-Saharan Africa | Malaria (mostly P. falciparum), tick-borne rickettsiae, acute schistosomiasis, filariasis |
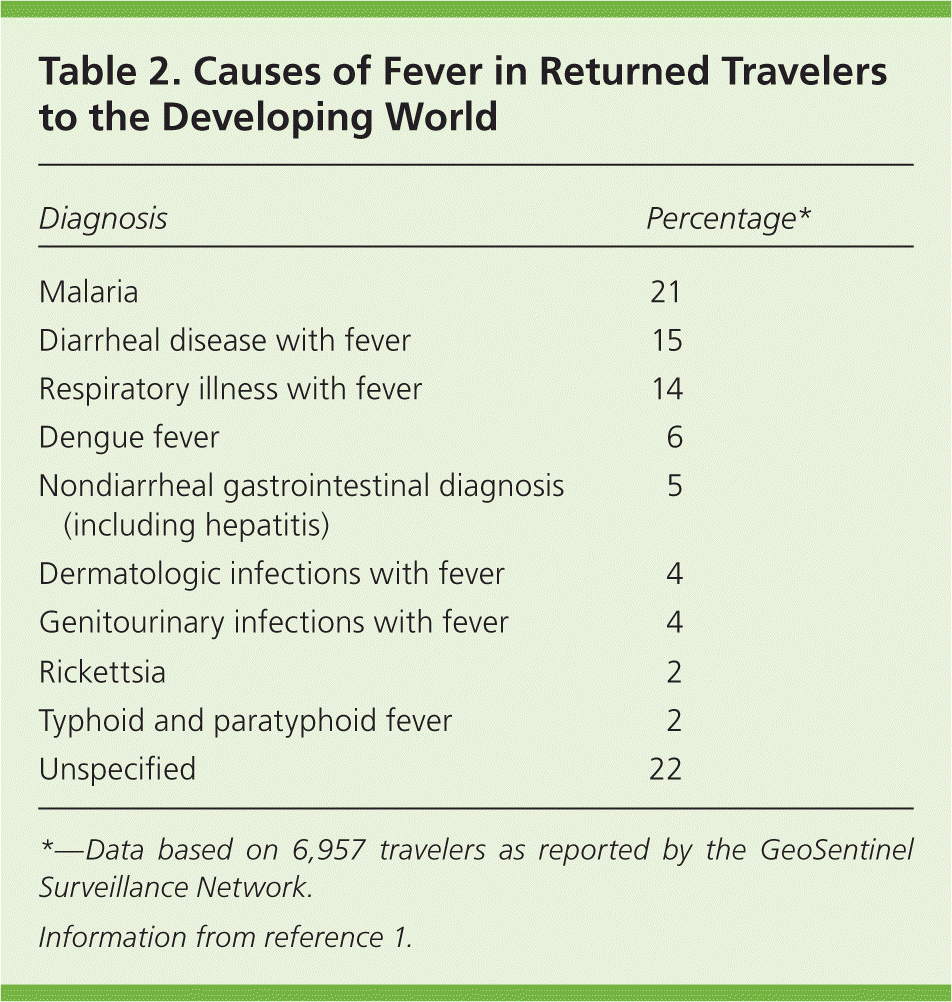
| Diagnosis | Percentage* |
|---|---|
| Malaria | 21 |
| Diarrheal disease with fever | 15 |
| Respiratory illness with fever | 14 |
| Dengue fever | 6 |
| Nondiarrheal gastrointestinal diagnosis (including hepatitis) | 5 |
| Dermatologic infections with fever | 4 |
| Genitourinary infections with fever | 4 |
| Rickettsia | 2 |
| Typhoid and paratyphoid fever | 2 |
| Unspecified | 22 |
The greatest challenge for the family physician is to identify the cause of fever without a focus. Table 3 lists the key elements of patient history for evaluating fever in returned travelers,4 and Table 4 lists the incubation periods for febrile tropical diseases.5 The physical examination should include a search for focal findings (Table 5).6–9 Dengue fever, malaria, and typhoid fever are common causes of fever without a focus in patients in whom a tropical infection is suspected. The physical findings with these infections are often nonspecific.4
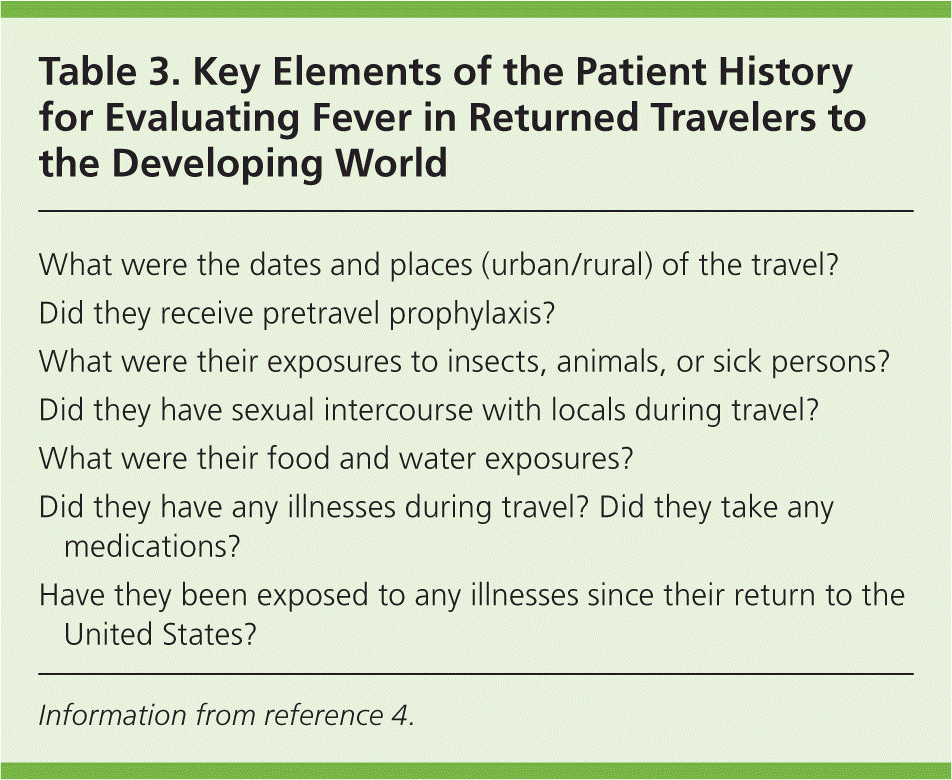
| What were the dates and places (urban/rural) of the travel? |
| Did they receive pretravel prophylaxis? |
| What were their exposures to insects, animals, or sick persons? |
| Did they have sexual intercourse with locals during travel? |
| What were their food and water exposures? |
| Did they have any illnesses during travel? Did they take any medications? |
| Have they been exposed to any illnesses since their return to the United States? |

| Disease | Usual incubation period (range) |
|---|---|
| ≤ 14 days | |
| Arbovirus encephalitis | 3 to 14 days (1 to 20 days) |
| Chikungunya | 2 to 4 days (1 to 14 days) |
| Dengue fever | 4 to 8 days (3 to 14 days) |
| Leptospirosis | 7 to 12 days (2 to 26 days) |
| Malaria (Plasmodium falciparum) | 6 to 30 days (weeks to months) |
| Malaria (Plasmodium vivax) | 8 to 30 days (months to years) |
| Spotted fever group rickettsiae | 3 to 21 days |
| Typhoid fever | 7 to 18 days (3 to 60 days) |
| > 14 days | |
| Amebic liver abscess | Weeks to months |
| Hepatitis A | 28 to 30 days (15 to 50 days) |
| Hepatitis E | 26 to 42 days (14 to 63 days) |
| Schistosomiasis (Katayama) | 28 to 56 days |
| Tuberculosis | Weeks to months to years |
| Visceral leishmaniasis | 2 to 10 months (10 days to years) |

| Physical examination | Diagnostic value |
|---|---|
| Abdomen | Liver tenderness occurs in hepatitis |
| Splenomegaly may occur in mononucleosis, malaria, visceral leishmaniasis (kala-azar), typhoid fever, and brucellosis | |
| Eyes | Conjunctivitis occurs in leptospirosis |
| Lymph nodes | Generalized lymphadenopathy may occur in typhoid fever, leptospirosis, brucellosis, dengue fever, and visceral leishmaniasis |
| Neurologic system | Altered mental status is a medical emergency and may occur in cerebral malaria or shock associated with hemorrhagic disease |
| Skin | Rose spots (clusters of 2- to 3-mm pink macules, usually on the trunk) suggest typhoid fever |
| Maculopapular rash occurs in leptospirosis and many rickettsial diseases | |
| Petechiae followed by purpura may occur in dengue fever, meningococcemia, and viral hemorrhagic fevers | |
| Vital signs | Pulse not increasing with fever spikes suggests typhoid fever or rickettsial disease |
The initial laboratory evaluation of the returned traveler with fever and no focus may include only a few laboratory tests (Table 6).5,10 Malaria can usually be diagnosed with a thin blood smear (utilizing the Giemsa stain). The Wright stain used in the manual differential of the complete blood count is almost as sensitive for detecting malaria parasites as the thin blood smear.10 Typhoid fever can be diagnosed with blood, urine, or stool cultures positive for Salmonella typhi. Dengue fever is a clinical diagnosis. Serologic testing for dengue virus immunoglobulin M and G and virus detection tests can be performed to confirm the diagnosis, but are not immediately available.3–8 Three vignettes of patients with travel-related fevers are presented below.
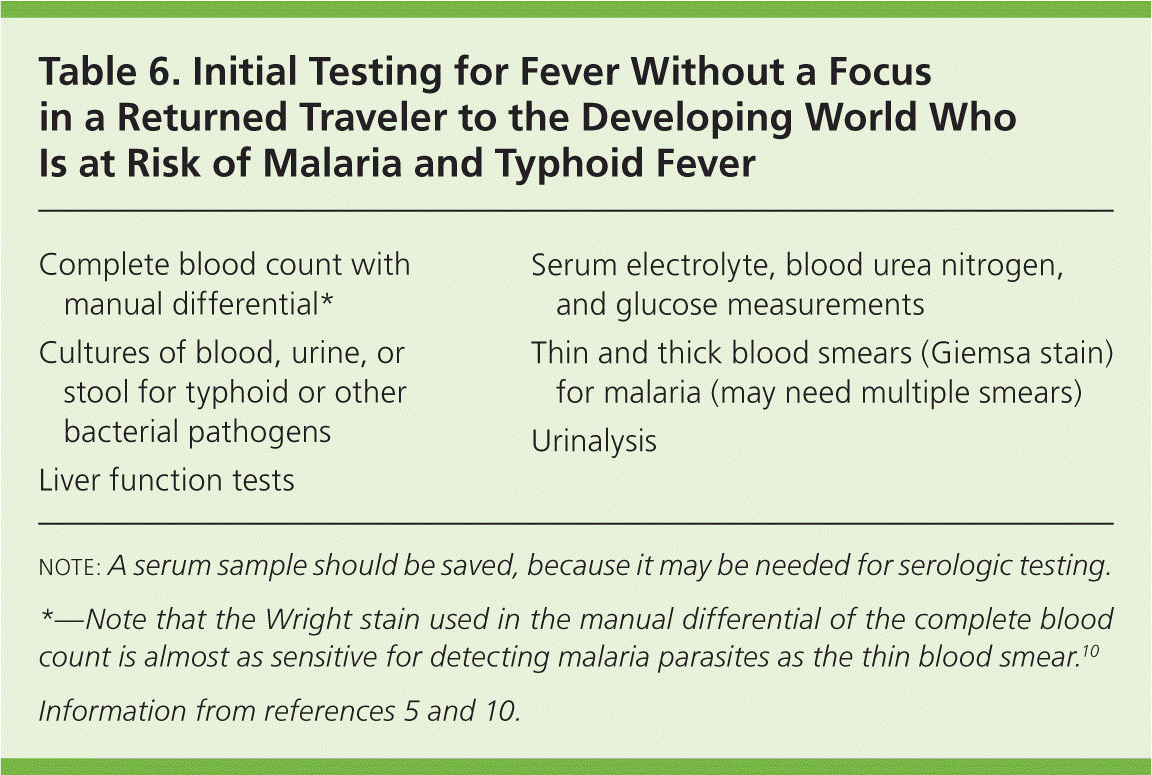
| Complete blood count with manual differential* |
| Cultures of blood, urine, or stool for typhoid or other bacterial pathogens |
| Liver function tests |
| Serum electrolyte, blood urea nitrogen, and glucose measurements |
| Thin and thick blood smears (Giemsa stain) for malaria (may need multiple smears) |
| Urinalysis |
Vignette 1
A 20-year-old college student spent a semester in rural Brazil. One week after his return, he developed headache, chills, and fever. Prior to traveling, he received typhoid fever and hepatitis A vaccines. He was prescribed mefloquine for malaria prophylaxis, but did not pick up the prescription. On the fifth day of fever, he was seen by his physician; his temperature was 103°F (39.4°C) and findings from an examination were normal. Results of laboratory tests (Table 65,10 ) were normal, except for low white blood cell and platelet counts (2,400 per mm3 [2.4 × 109 per L] and 106 × 103 per mm3 [106 × 109 per L], respectively). A thin blood smear and blood culture for malaria were negative. Over the next week, his fever and chills continued.
Outcome and Discussion
On the 15th day of fever, the laboratory tests were repeated. This time, the thin blood smear was positive for Plasmodium falciparum malaria, with less than 1% parasitemia. He was treated as an outpatient with atovaquone/proguanil (Malarone) and was afebrile on the third day of therapy.
The patient's history was consistent with malaria or typhoid fever. He had a history of prolonged travel to an area where both diseases were endemic, had not taken malaria prophylaxis, and had a prolonged illness characterized by high fever, chills, malaise, and headache. Malaria should be suspected in patients with spiking fevers, leukopenia, thrombocytopenia, and increased bands who have visited a malaria-endemic area and did not take prophylaxis.11 Patients with a low parasitemia may need multiple blood smears to produce a positive result. A thin blood smear consists of a Giemsa stain performed on a thin smear of blood that is fixed with alcohol so that the red blood cells remain intact. If the results of the thin blood smear are negative, then a thick blood smear should be performed. A thick blood smear consists of a Giemsa stain performed on dry blood that is not fixed so that the red blood cells lyse. In the developing world, if malaria blood smears cannot be reliably performed, then rapid diagnostic tests that detect specific circulating malaria antigens may be used.12,13
Four species of Plasmodium can infect humans: P. falciparum, Plasmodium vivax, Plasmodium malariae, and Plasmodium ovale.14 A fifth Plasmodium species, Plasmodium knowlesi, is an emerging human pathogen that may be mistaken microscopically for P. malariae. Infection with P. knowlesi may occur following exposure to macaque monkeys.15 Malaria is acquired by bites from Anopheles mosquitoes, which are dusk to dawn feeders (Figure 1). Malaria sporozoites from the mosquito travel to the human liver, where they develop into merozoites and are released in the blood weeks to months later. The merozoites feed on hemoglobin and rupture the red blood cells in 48 hours (for P. falciparum, P. vivax, and P. ovale) or 72 hours (for P. malariae). P. vivax and P. ovale can remain latent in the liver for months or years, and P. malariae can remain latent in red blood cells for months or years. When red blood cells rupture, fever spikes occur. If the rupture happens synchronously, then there would be a tertian fever (recurring every third day) or quartan fever (recurring every fourth day). However, red blood cell rupture usually happens asynchronously, and the fever spikes are usually random.13,15–17
P. falciparum is the most common species that infects humans and is the only malaria species that causes fulminant disease, due to high parasitemias and the ability to bind to capillaries and clog the microcirculation. Each year there are more than 250 million cases of malaria worldwide.10 Malaria, human immunodeficiency virus/AIDS, and tuberculosis are the leading infectious causes of deaths in the world; each is responsible for approximately 1 to 2 million deaths annually.18 Persons living in malaria-endemic countries develop partial immunity; however, if these persons immigrate to the United States, this immunity rapidly wanes. Approximately 18 million U.S. travelers visit malaria-endemic countries each year, and about 1,500 contract malaria.12–17
Findings in patients with P. falciparum malaria often include fever, headache, malaise, arthralgias/myalgias, nausea/vomiting, and abdominal pain. Splenomegaly or jaundice occurs in less than 35% of patients. Laboratory tests may show anemia (29%), thrombocytopenia (45%); white blood cell count of less than 5,000 per mm3 (5 × 109 per L; 26%); elevated bands (85%); positive thin blood smear (99%); and negative thin blood smear with positive thick blood smear (1%).10 Rarely, P. falciparum can cause severe hemolysis with hemoglobinuria (also called blackwater fever) and dark red urine.10,13
More than 75% of U.S. travelers with malaria do not take adequate prophylaxis.4 This might be because of the cost or fear of adverse effects, which include insomnia and vivid dreams with mefloquine. Chemoprophylaxis and diethyltoluamide (DEET)-based mosquito repellants are effective in preventing malaria infection. Persons traveling outside of the United States should use repellents that contain 30% to 50% DEET.19 Current guidelines for the treatment of malaria are available from the Centers for Disease Control and Prevention (http://www.cdc.gov/malaria). Chloroquine (Aralen) is the treatment of choice for P. falciparum in areas where there is no resistance. Other medications, such as quinine plus tetracycline or mefloquine, are effective in chloroquineresistant areas.4,10,13,17
Vignette 2
A seven-year-old Pakistani boy born in the United States visited Pakistan for six weeks with his father. Neither the father nor the boy sought pretravel vaccines or prophylaxis. Fourteen days after returning to the United States, the child developed fever (104°F [40°C]) without a focus. On day 6 of the child's fever, the father initiated a 10-day course of amoxicillin using an old prescription. The fever stopped during the course of amoxicillin, but recurred a few days later, prompting a hospital visit.
Outcome and Discussion
This case is a good example of how families visiting their homeland may not believe they can get a tropical infection. The child was seen by a physician on the 23rd day of illness; at this time his temperature was 103°F (39.4°C) and findings from an examination were normal. There was no history of diarrhea. Results of laboratory tests, including multiple malaria blood smears, were negative. Stool and urine cultures were negative for other bacterial pathogens; however, his blood culture was positive for S. typhi sensitive to amoxicillin, ciprofloxacin (Cipro), and trimethoprim/sulfamethoxazole (TMP/SMX). The patient was treated with TMP/SMX for 14 days. He was afebrile in 48 hours.
Salmonella infections are usually acquired via the food chain. Had the child been vaccinated against typhoid fever and avoided certain foods/liquids, this infection may not have occurred.4 When traveling outside the United States, it is usually safe to consume bottled water; hot cooked food; dry food; and fruits and vegetables that can be peeled. Tap water; ice; fresh juices and salads; unpasteurized dairy products; uncooked sauces and toppings; open buffets; and food sold by street vendors should be avoided.4
Enteric fever includes S. typhi (typhoid fever) and Salmonella paratyphi (paratyphoid fever) infections. Most enteric fever cases worldwide are typhoid fever. As a result of safer water and food, typhoid fever cases in the United States fell from 35,994 cases in 1920 to about 500 cases per year beginning in 1990. Overall, 80% of the cases in 1990 occurred in travelers. Worldwide, 16 million cases of typhoid fever occur annually, resulting in 600,000 deaths. Asia has the greatest disease burden, with 13 million cases.20,21
Patients with typhoid fever present in a similar fashion to those with malaria. After an incubation period of three to 60 days, the first manifestations of typhoid fever are fever and malaise. The fever increases in a stepwise fashion during the first week, then becomes sustained at 102.5°F to 105°F (39.2°C to 40.6°C) for the next week. Associated symptoms include anorexia, vomiting, and abdominal pain. Diarrhea is more likely in children, whereas constipation is more likely in adults; either occurs in less than one-half of patients. Signs of typhoid fever include rose spots, hepatosplenomegaly, and relative bradycardia with fever spikes. Rose spots, which are easily seen in fair-skinned persons, are maculopapular, pink-colored lesions on the trunk that occur in 5% to 30% of patients. Laboratory tests are nonspecific; findings include leukopenia, thrombocytopenia, elevated erythrocyte sedimentation rate/C-reactive protein level, and elevated liver enzyme level. Typhoid fever should be suspected in patients with spiking fever and leukopenia who have visited a typhoid-endemic area and who have not received a typhoid fever vaccination.21 Blood cultures are positive in 40% to 60% of patients.20,21 Sometimes urine or stool cultures are positive for S. typhi when blood cultures are negative. The most sensitive way to recover S. typhi is by bone marrow culture, but this is rarely used in routine clinical practice.20,21
S. typhi is becoming increasingly resistant to antibiotics. Amoxicillin and TMP/SMX resistance is common, and ciprofloxacin resistance is becoming more common. Alternatives for oral therapy include cefixime (Suprax) or azithromycin (Zithromax). Amoxicillin is associated with a relapse rate of 4% to 8%, which happened to the patient in vignette 2.20–22 Ciprofloxacin is the drug of choice for empiric typhoid fever treatment, unless local fluoroquinolone resistance is high, such as in the Indian subcontinent. In this case, an injectable third-generation cephalosporin should be the empiric antibiotic choice.5
Vignette 3
A 15-year-old male developed fever two days after his return from the Dominican Republic. He also had malaise, body aches, headache, nausea, and abdominal pain. On day 6 of fever, he developed a generalized rash, gingival bleeding, melena, and bloody emesis, and was hospitalized.
Outcome and Discussion
On admission, the patient's vital signs were normal except for a temperature of 100.5°F (38.1°C). His physical examination revealed abdominal tenderness, nonpitting leg edema, and petechiae of the palate and legs. Findings on laboratory tests included hemoconcentration (53% hematocrit) and thrombocytopenia (platelet count of 14 × 103 per mm3 [14 × 109 per L]). The patient was treated with intravenous fluids and ceftriaxone (Rocephin) for possible meningococcal sepsis. By day 4 of hospitalization, the patient's symptoms had resolved and results of his laboratory tests were normal. Blood smears and cultures for malaria were negative. Results of serologic testing (enzyme-linked immunosorbent assay) for dengue virus immunoglobulin M (IgM) and IgG were positive. The patient had dengue hemorrhagic fever.
Dengue fever should be suspected in patients with spiking fevers and leukopenia who have visited a dengue-endemic area.23–25 The history of travel has to be within 14 days. The incubation period is typically four to eight days (range of three to 14 days). Dengue fever is transmitted by the day-biting Aedes mosquito (Figure 2). Four different serotypes of dengue virus (DENV 1, DENV 2, DENV 3, and DENV 4) exist. Infection and immunity to one serotype may predispose to more serious disease if infected with another serotype. Dengue is an arbovirus; the more than 500 arboviruses are transmitted by the bites of mosquitoes, ticks, and sand flies. Dengue virus is in the Flavivirus genus, which includes West Nile and yellow fever viruses. Dengue fever is endemic in more than 100 tropical countries, with 40% of the world's population. The global incidence of dengue infection is 50 to 100 million cases each year, resulting in 500,000 hospitalizations and 12,500 deaths.23–25 A few hundred U.S. travelers develop dengue fever each year.23,24,26–29 The clinical presentations of dengue and yellow fever are similar, except yellow fever is often associated with hyperbilirubinemia and clinical jaundice. Globally, yellow fever infects about 200,000 persons each year and is fatal in about 30,000. Yellow fever is rare in returned U.S. travelers, with about one case reported every five years.30
Patients with dengue fever may be asymptomatic or may have a mild undifferentiated febrile illness. Classic dengue fever lasts fewer than seven days. Symptoms include fever, retro-orbital headache, muscle or joint pain, fine maculopapular rash, nausea, and vomiting. In about 1% of patients, dengue fever is severe and manifests as dengue hemorrhagic fever or dengue shock syndrome.23 Dengue hemorrhagic fever is defined by the triad of hemorrhagic manifestations, thrombocytopenia, and plasma leakage (increased hematocrit level, hypoproteinemia, or effusions). The mortality rate in dengue shock syndrome can be 10% to 20%.28,29 Dengue shock syndrome is characterized by a narrow pulse pressure (less than 20 mm Hg) or hypotension. Dengue hemorrhagic fever and dengue shock syndrome do not occur with the first dengue infection, but may occur on reinfection with a different dengue serotype.23,25,28 Antibodies from the first infection may inhibit an adequate antibody response to the second infection. Infants born with their mother's antibodies to one serotype can develop dengue hemorrhagic fever or dengue shock syndrome if infected with another serotype.23,24,26–29
Laboratory tests for a suspected case of dengue fever include a complete blood count evaluating for leukopenia, thrombocytopenia, and an increased hematocrit level. A tourniquet test involves inflating the blood pressure cuff midway between systolic and diastolic for five minutes. Having more than 20 petechiae per inch of skin is a positive result and can be used to diagnose increased capillary fragility or thrombocytopenia. Dengue fever is usually confirmed by at least a fourfold increase in reciprocal IgG dengue antibody titers in acute/convalescent serum samples. The presence of dengue-specific IgM antibodies is presumptive evidence of acute dengue infection. The most important treatment for dengue fever is bed rest and adequate hydration. Affected patients should avoid aspirin and nonsteroidal anti-inflammatory drugs because of their anticoagulant properties.23,24,26–29 For prevention, there is no medication prophylaxis or vaccine available. The best prevention method is to reduce or avoid mosquito bites by using repellents (containing 30% to 50% DEET19), wearing protective clothing, and using bed nets.23
Data Sources: A PubMed search was completed using the key words malaria, dengue, typhoid, and fever in travelers. Also reviewed were the following journals over the past 15 years: Clinical Infectious Diseases, Lancet Infectious Diseases, New England Journal of Medicine, Morbidity and Mortality Weekly Report, and Emerging Infectious Diseases. Search dates: January 2011 through September 2011.
