
Am Fam Physician. 2014;89(3):153-158
Related Practice Guideline: ACIP Releases 2014 Child and Adolescent Immunization Schedules.
Author disclosure: No relevant financial affiliations.
Recommending and providing childhood vaccines is a vital part of a family physician's role in delivering health care to our youth. This editorial reviews several changes in childhood vaccines in the past two years (Table 1).
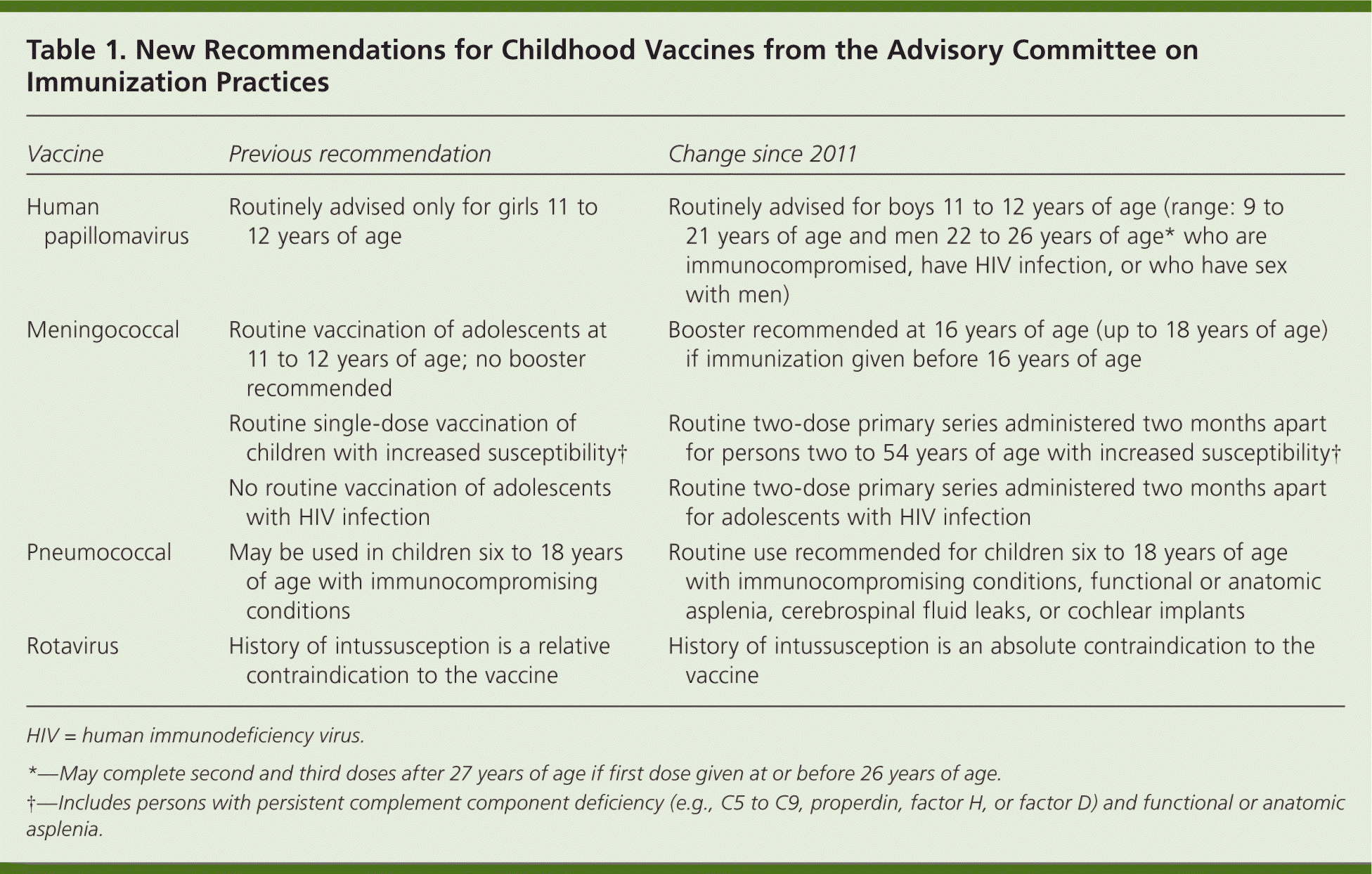
| Vaccine | Previous recommendation | Change since 2011 |
|---|---|---|
| Human papillomavirus | Routinely advised only for girls 11 to 12 years of age | Routinely advised for boys 11 to 12 years of age (range: 9 to 21 years of age and men 22 to 26 years of age* who are immunocompromised, have HIV infection, or who have sex with men) |
| Meningococcal | Routine vaccination of adolescents at 11 to 12 years of age; no booster recommended | Booster recommended at 16 years of age (up to 18 years of age) if immunization given before 16 years of age |
| Routine single-dose vaccination of children with increased susceptibility† | Routine two-dose primary series administered two months apart for persons two to 54 years of age with increased susceptibility† | |
| No routine vaccination of adolescents with HIV infection | Routine two-dose primary series administered two months apart for adolescents with HIV infection | |
| Pneumococcal | May be used in children six to 18 years of age with immunocompromising conditions | Routine use recommended for children six to 18 years of age with immunocompromising conditions, functional or anatomic asplenia, cerebrospinal fluid leaks, or cochlear implants |
| Rotavirus | History of intussusception is a relative contraindication to the vaccine | History of intussusception is an absolute contraindication to the vaccine |
In 2013, the Advisory Committee on Immunization Practices (ACIP) recommended combining the immunization schedules for children six years and younger and for children seven through 18 years of age into one schedule for all children from birth through 18 years of age. Printable versions of this document with regular and catch-up schedules are available at https://www.aafp.org/patient-care/immunizations/schedules.html.
This new easy-to-read schedule illustrates the 2011 ACIP recommendation that boys 11 to 12 years of age should routinely receive the quadrivalent human papillomavirus (HPV) vaccine in a three-dose series; the series can start as early as nine years of age. The vaccine is also routinely recommended for men 22 to 26 years of age who are immunocompromised, including those with human immunodeficiency virus infection, and for men who have sex with men. This vaccine covers HPV strains 16 and 18, which are responsible for about 70% of cervical and other genital cancers and many oropharyngeal cancers; and strains 6 and 11, which are known to be largely responsible for most genital warts and recurrent respiratory papillomatosis. HPV is increasingly linked to head and neck cancers, and males are at higher risk than females.1 We should remind ourselves, and our patients, that in targeting HPV, vaccination prevents cancer. For parents concerned about how the vaccine affects sexual behavior, research has shown that there is no increase in sexual activity in girls 11 to 12 years of age followed over four years after receiving the HPV vaccination.2
Another 2011 ACIP recommendation updates the contraindications for the rotavirus vaccine: a history of intussusception, formerly a precaution, is now cited as an absolute contraindication to vaccination. For all others without a history of intussusception, there is a small overall excess risk of approximately 0.79 intussusception events for every 100,000 vaccinated infants. This translates to 33 excess intussusception events annually in the United States after rotavirus vaccination. When compared with the 40,000 hospitalizations prevented annually since the rotavirus vaccine was introduced, it appears to be a small and acceptable public health risk.3
Also in the past two years, ACIP made two new recommendations concerning quadrivalent meningococcal conjugate vaccines: (1) routine vaccination of adolescents at 11 or 12 years of age with a booster dose at 16 years of age (up to 18 years of age); and (2) a two-dose primary series administered two months apart for persons two through 54 years of age with persistent complement component deficiency (e.g., C5 to C9, properdin, factor H, or factor D) and functional or anatomic asplenia, and for adolescents with human immunodeficiency virus infection.4
ACIP also recommends routine use of the 13-valent pneumococcal conjugate vaccine for children six to 18 years of age with immunocompromising conditions, functional or anatomic asplenia, cerebrospinal fluid leaks, or cochlear implants who have not previously received the vaccine. These children should receive the 13-valent pneumococcal conjugate vaccine regardless of whether they received the 7-valent pneumococcal conjugate vaccine or the 23-valent pneumococcal polysaccharide vaccine.5
