
Am Fam Physician. 2014;89(10):803-811
A more recent article on neglected parasitic infections is available.
Author disclosure: No relevant financial affiliations.
Neglected parasitic infections, including Chagas disease, toxocariasis, cysticercosis, and toxoplasmosis, affect millions of persons in the United States. Relatively few resources have been devoted to surveillance, prevention, and treatment of these diseases. Chagas disease primarily affects Latin American immigrants and can cause heart failure and death if not treated. Immediate antiparasitic treatment is indicated for most patients with acute Chagas disease. Treatment is recommended for patients younger than 18 years who have chronic Chagas disease and is generally recommended for adults younger than 50 years who do not have advanced cardiomyopathy; treatment decisions for other patients should be made on an individual basis. Toxocariasis primarily affects children and can cause gastrointestinal, respiratory, and ophthalmologic disease. Treatment options include albendazole and mebendazole. Patients with ocular infection require referral to an ophthalmologist. Neurocysticercosis, a form of cysticercosis, is the most common infectious cause of seizures in some parts of the United States. Initial treatment should focus on symptom control. Humans generally acquire toxoplasmosis by eating undercooked contaminated meat or ingesting things that have been contaminated with cat feces. Congenital infection can result in miscarriage or adverse fetal effects. Treatment is recommended for immunosuppressed persons, pregnant women, and immunocompetent persons with severe symptoms.
Neglected parasitic infections, including Chagas disease, toxocariasis, cysticercosis, and toxoplasmosis, can cause severe illness, but limited resources have been devoted to better understanding their impact and burden. Physicians may not be familiar with these infections because their clinical presentation, diagnosis, and treatment are typically not emphasized during medical training. However, it is crucial for family physicians to understand the basic principles of diagnosis and treatment of these diseases. A summary of the key points about epidemiology, clinical manifestations, diagnostic evaluation, and treatment for each disease is presented in Table 1.
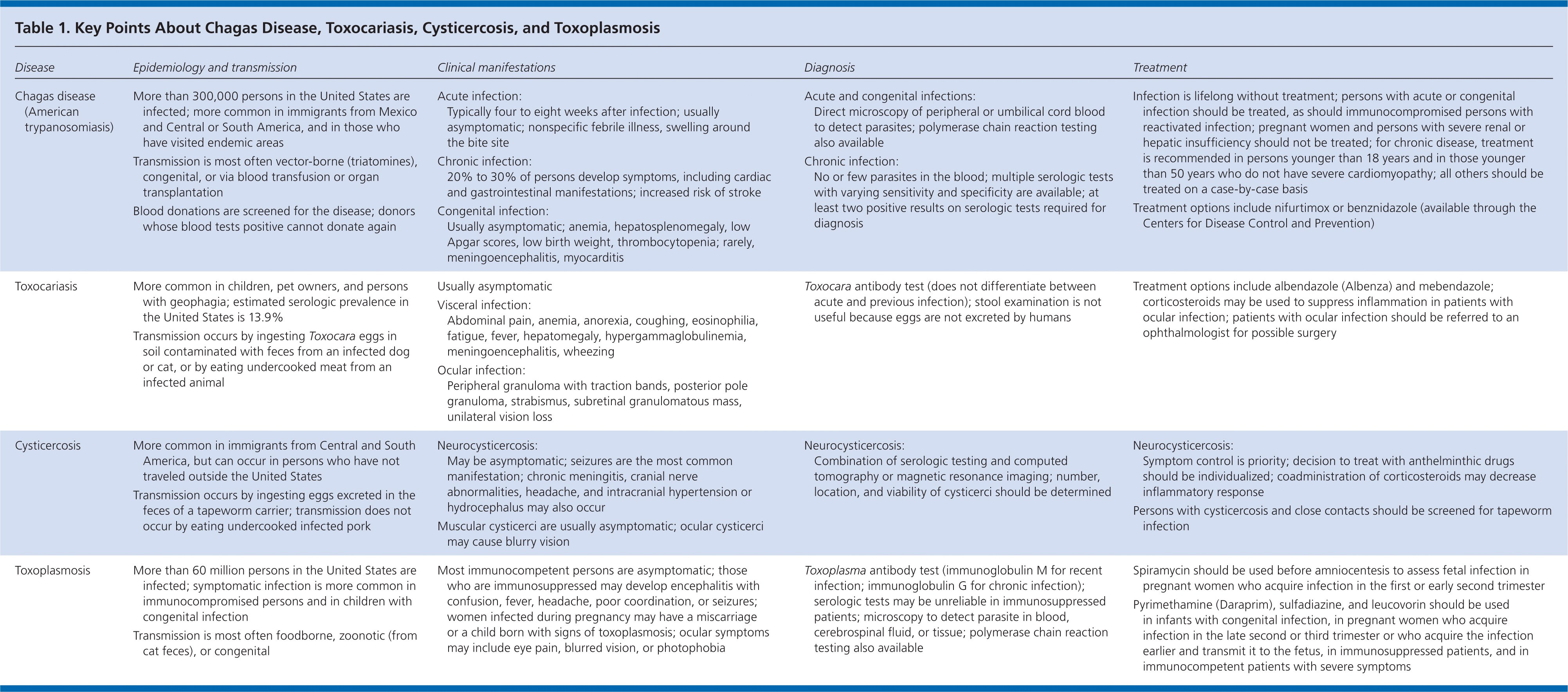
| Disease | Epidemiology and transmission | Clinical manifestations | Diagnosis | Treatment | |||
|---|---|---|---|---|---|---|---|
| Chagas disease (American trypanosomiasis) |
| Acute infection:
| Acute and congenital infections:
|
| |||
| Toxocariasis |
|
| Toxocara antibody test (does not differentiate between acute and previous infection); stool examination is not useful because eggs are not excreted by humans | Treatment options include albendazole (Albenza) and mebendazole; corticosteroids may be used to suppress inflammation in patients with ocular infection; patients with ocular infection should be referred to an ophthalmologist for possible surgery | |||
| Cysticercosis |
| Neurocysticercosis:
| Neurocysticercosis:
| Neurocysticercosis:
| |||
| Toxoplasmosis |
| Most immunocompetent persons are asymptomatic; those who are immunosuppressed may develop encephalitis with confusion, fever, headache, poor coordination, or seizures; women infected during pregnancy may have a miscarriage or a child born with signs of toxoplasmosis; ocular symptoms may include eye pain, blurred vision, or photophobia | Toxoplasma antibody test (immunoglobulin M for recent infection; immunoglobulin G for chronic infection); serologic tests may be unreliable in immunosuppressed patients; microscopy to detect parasite in blood, cerebrospinal fluid, or tissue; polymerase chain reaction testing also available |
| |||
Chagas Disease
Chagas disease, also known as American trypanosomiasis, is caused by the parasite Trypanosoma cruzi. Transmission to humans occurs mainly through contact with insects. Infection occurs when an infected triatomine defecates after a blood meal and the feces, which contains the parasite, is rubbed into the bite wound or mucous membranes (Figure 1).1 Transmission can also occur congenitally or via blood transfusion, organ transplantation, contaminated food, or laboratory exposure. Chagas disease is endemic throughout Mexico and Central and South America, where an estimated 8 to 11 million persons are infected.2 More than 300,000 persons in the United States are thought to be infected,2 most of whom acquired the disease in Latin America. However, infected triatomines have been found in the United States, and domestic vector-borne transmission has occurred.3
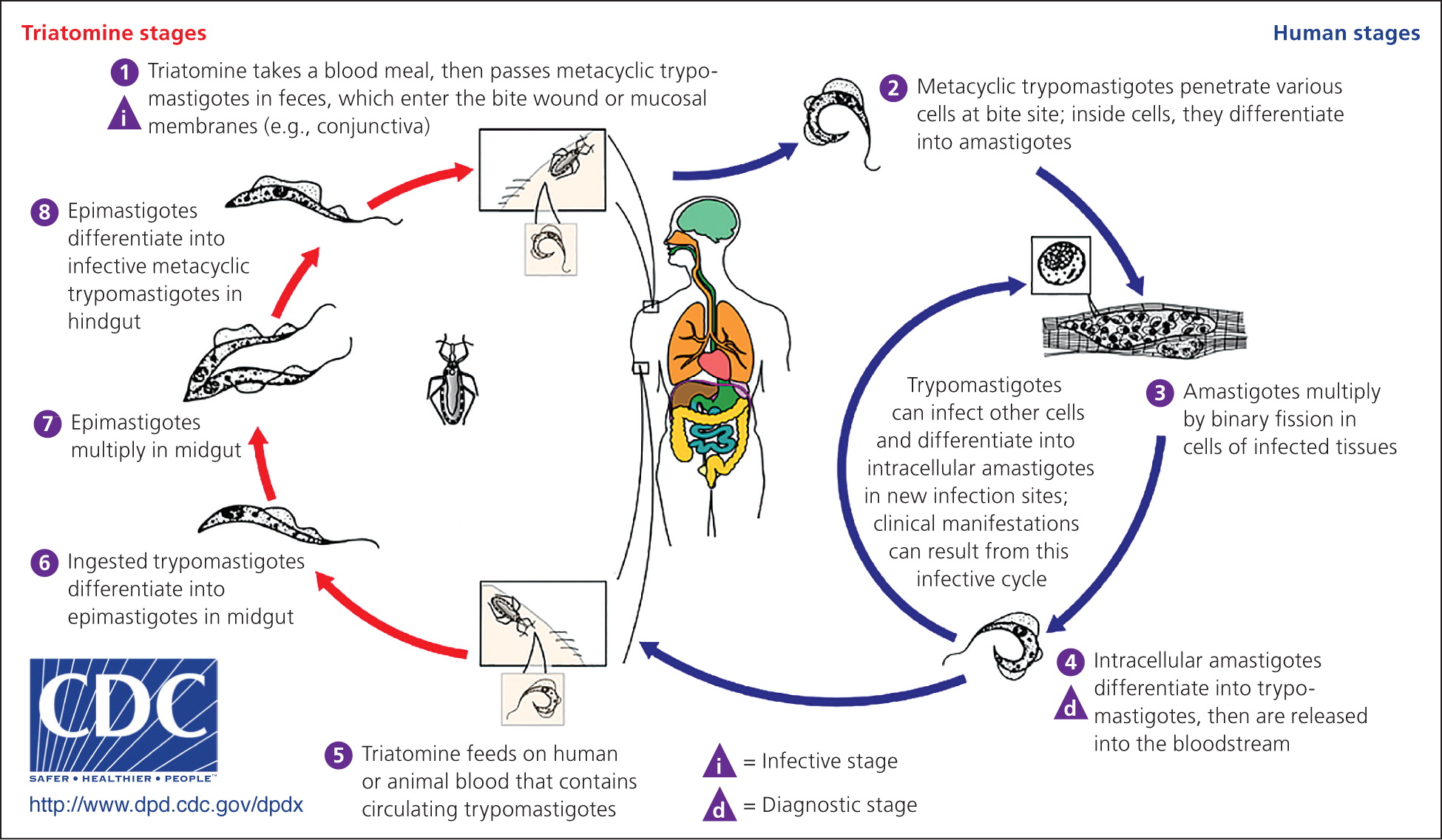
There are two phases of the disease: acute, which lasts for weeks or months after the initial infection, and chronic. Infection is lifelong in the absence of treatment. Clinical manifestations are often mild or absent in the acute phase; swelling around the bite site may be present. If the inoculation site is the conjunctiva, unilateral palpebral edema may occur. Most patients with chronic Chagas disease remain asymptomatic, but 20% to 30% of persons with the infection develop clinical manifestations that can be life-threatening.4 Cardiac disease, including conduction abnormalities, apical aneurysm, or heart failure, may occur. Gastrointestinal manifestations include megaesophagus or megacolon. Stroke risk is increased in patients infected with T. cruzi. Congenitally infected infants are often asymptomatic; some may have low birth weight or low Apgar scores, or may develop anemia, thrombocytopenia, or hepatosplenomegaly. Rarely, myocarditis or meningoencephalitis may occur in infants with congenital infection.
Acute or congenital Chagas disease is diagnosed by detection of parasites via direct microscopy of anticoagulated cord blood or peripheral blood; polymerase chain reaction testing may also be used to detect acute infection. After the acute phase, few or no parasites are present; serologic testing for antibodies to T. cruzi is required to diagnose chronic Chagas disease. Because no single serologic test has sufficient sensitivity and specificity to confirm the diagnosis, positive results on at least two different tests are required for diagnosis. Since 2007, U.S. blood donors have been screened for T. cruzi infection. Those with positive results can no longer donate blood, regardless of confirmatory test results, and are advised to consult with their physician for further workup and confirmation of infection.
Patients with newly diagnosed Chagas disease should have a physical examination with electrocardiography. Children whose mothers have Chagas disease should also be tested. Immediate antiparasitic treatment is indicated for patients with acute disease, including those with congenital infection and immunocompromised patients with reactivated disease.5 Pregnant women and patients with severe hepatic or renal insufficiency should not be treated because of adverse drug effects. Treatment is recommended for patients younger than 18 years who have chronic Chagas disease and is generally recommended for adults younger than 50 years who do not have advanced cardiomyopathy; treatment decisions for other patients should be made on an individual basis.2,5 Benznidazole and nifurtimox are used to treat Chagas disease. Neither drug is approved by the U.S. Food and Drug Administration, but they are available through the Centers for Disease Control and Prevention under investigational protocols.5 A treatment course ranges from 60 to 90 days, and adverse effects include weight loss, anorexia, and polyneuropathy.
Toxocariasis
Toxocariasis is caused by the roundworms Toxocara canis and Toxocara cati and occurs anywhere dogs or cats are present. After an infected dog or cat passes Toxocara eggs in its feces, it takes about two to four weeks for larvae to develop and for the eggs to become infectious; humans become infected by ingesting Toxocara eggs or by eating undercooked meat from an animal infected with Toxocara larvae (Figure 2).6 The seroprevalence of Toxocara infection in the United States is estimated to be 13.9%; however, the proportion of persons who have clinical toxocariasis is unknown.7 Studies have shown soil samples to have contamination rates as high as 40%, especially in areas frequented by dogs and cats (e.g., sandboxes, playgrounds).8 Children are at high risk of infection because they often visit these areas and ingest dirt as a result of their play habits and hygiene. Geophagia (i.e., the deliberate consumption of soil) and owning a dog or cat are other factors that increase the risk of infection. The development of clinical disease depends on the parasite load, the host's immune response, and the migration path of the larvae; most patients infected with Toxocara remain asymptomatic.
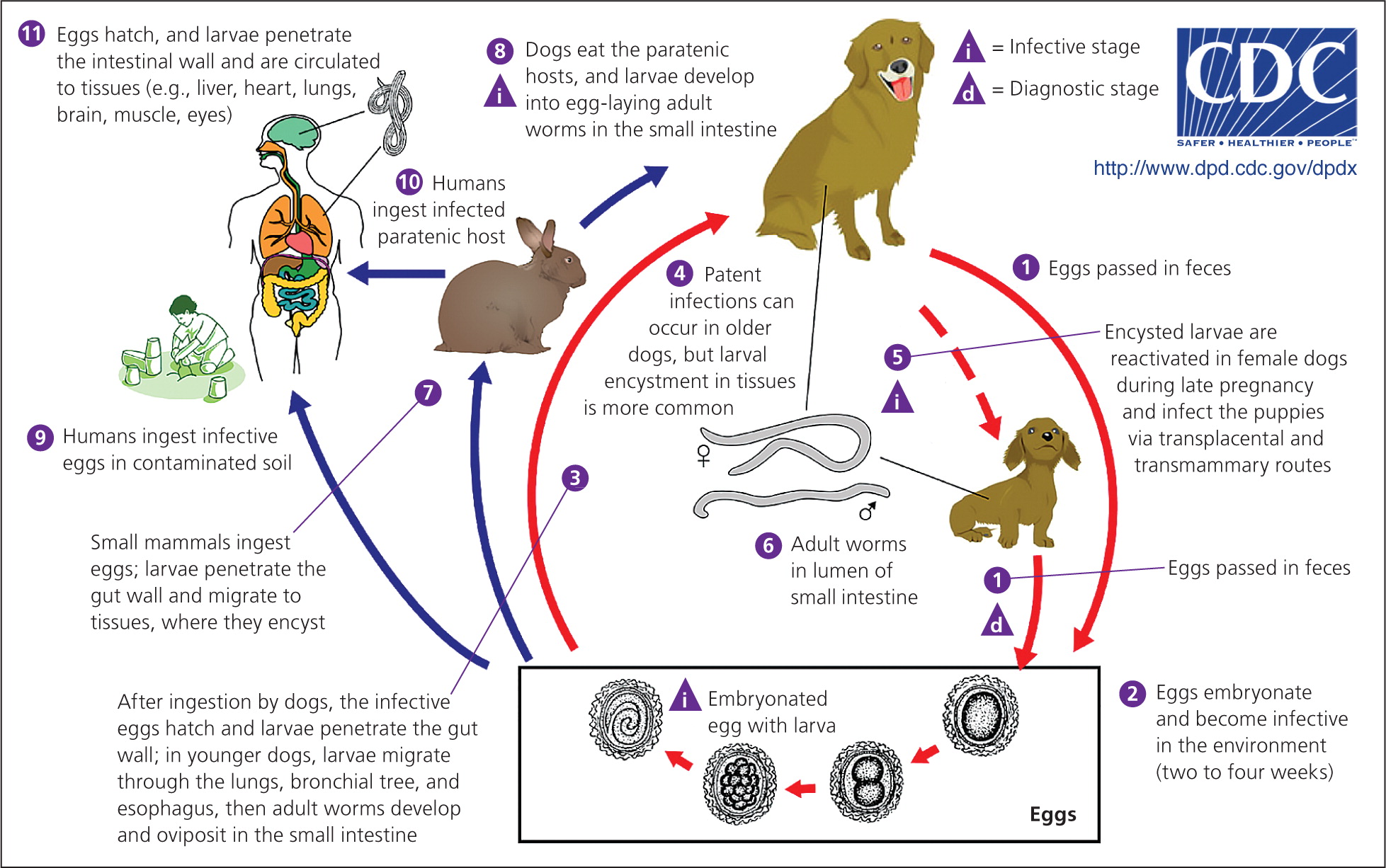
Three clinical manifestations of toxocariasis have been described: visceral, ocular, and covert toxocariasis (also called common toxocariasis). Visceral toxocariasis is typically diagnosed in young children (mean age of two to four years) and is characterized by a marked inflammatory response to larvae migrating to the liver or other tissues. Signs and symptoms are often nonspecific and may include fever, wheezing, coughing, abdominal pain, hepatomegaly, meningoencephalitis, anorexia, and fatigue. Laboratory findings often include eosinophilia; anemia or hypergammaglobulinemia may also be present. Ocular toxocariasis occurs when a Toxocara larva migrates to the eye, resulting in inflammation and scarring that can lead to vision loss. It typically occurs in slightly older children (mean age of five to eight years). Common ophthalmologic findings include subretinal granulomatous mass, posterior pole granuloma, unilateral vision loss, strabismus, and peripheral granuloma with traction bands; usually, only one eye is affected. Patients with ocular toxocariasis often have a normal eosinophil count. In covert toxocariasis, symptoms are mild and nonspecific.
Serologic testing for Toxocara antibodies is available, although a positive result does not differentiate between acute and previous infection. Stool examination is not helpful because Toxocara larvae do not mature into adult worms that excrete eggs in humans.
Treatment options for toxocariasis include albendazole (Albenza) and mebendazole. A five-day course is generally sufficient, although there is a lack of data regarding optimal treatment duration.9 Corticosteroids should be prescribed if evidence of inflammation is present; this is especially important to prevent scarring in patients with ocular toxocariasis, which may lead to permanent vision loss. Patients with ocular infection require close follow-up with an ophthalmologist, and surgery may be warranted. Strategies to prevent Toxocara infection include prompt disposal of pet feces, routine deworming of pets, and hygiene techniques such as hand washing and teaching children not to eat dirt.
Cysticercosis
Cysticercosis, a tissue infection with encysted Taenia solium larvae, is acquired by ingesting eggs excreted in the feces of a human carrier of a pork tapeworm (Figure 3).10 Taeniasis, an intestinal infection with the adult T. solium tapeworm, is acquired when a human ingests undercooked pork infected with cysticerci, or larval cysts. Persons with taeniasis shed tapeworm eggs in the feces, which can contaminate the environment in settings where personal hygiene practices are poor. Once the infectious eggs are ingested, they migrate into tissues and develop into cysticerci. In the United States, the highest prevalence of cysticercosis occurs in immigrants from Central and South America who acquired the infection in their home country; however, up to 15% of patients in a study of cysticercosis-associated deaths were U.S. natives.11 Having a household contact with taeniasis increases the risk of cysticercosis.
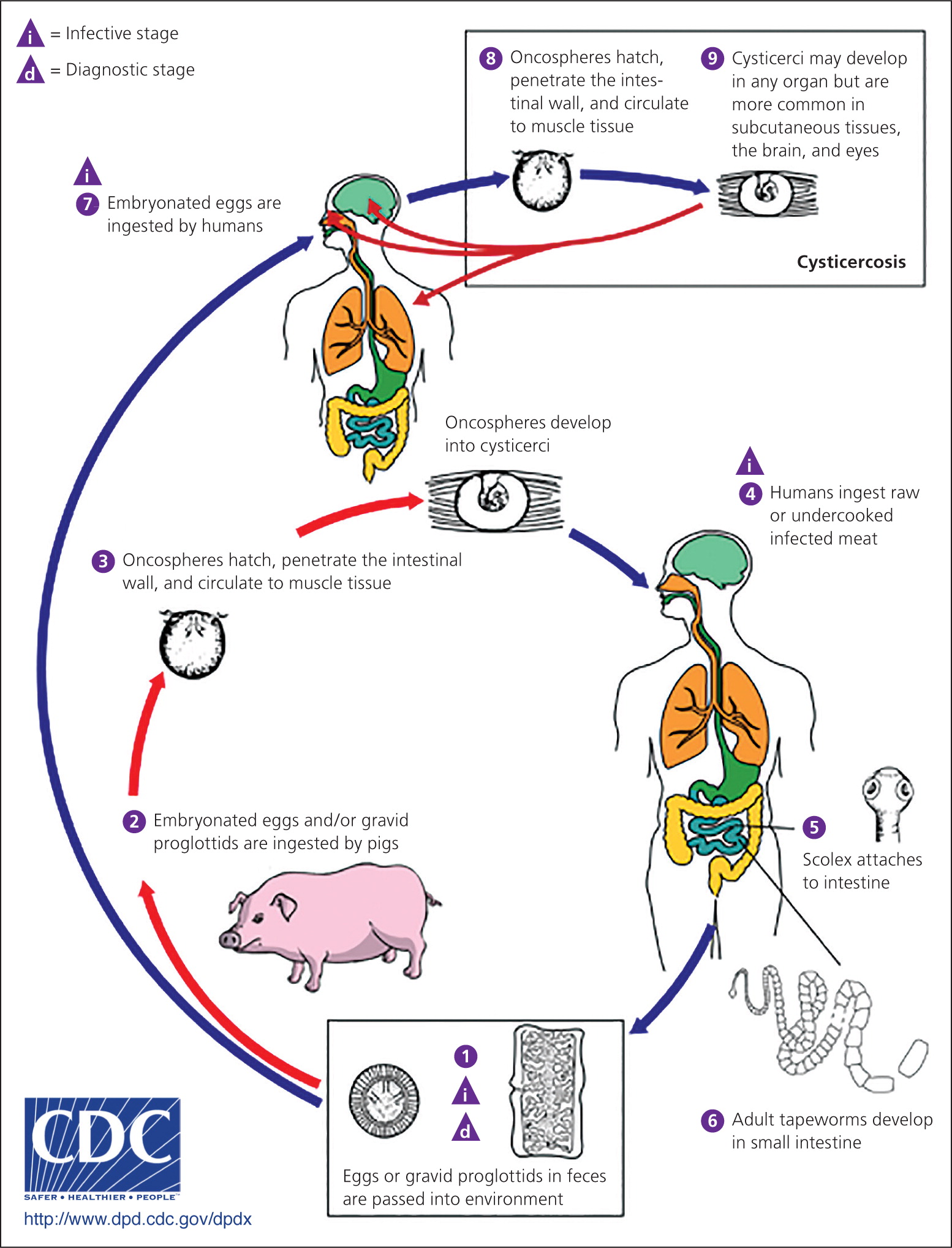
Cysticerci can develop in the muscles, eyes, brain, and spinal cord; symptom onset varies from months to years from time of initial infection. Symptoms are most often caused by the inflammatory response to dying parasites, although they can be caused by mass effect. Subcutaneous and muscular cysticerci are generally asymptomatic, although lumps may develop under the skin. Neurocysticercosis occurs when cysticerci invade the central nervous system. The most common clinical manifestations are seizures, which occur in 50% to 80% of symptomatic patients with parenchymal cysts, and intracranial hypertension or hydrocephalus, which occurs in 20% to 30% of symptomatic patients.12 Other manifestations include headache, chronic meningitis, and cranial nerve abnormalities. Parenchymal disease with few cysts has a better long-term prognosis than extraparenchymal disease or infection with large numbers of cysts.
A combination of serologic testing and neuroimaging with computed tomography or magnetic resonance imaging is typically required for diagnosis. An enzyme-linked immunoelectrotransfer blot is the preferred serologic test because of its sensitivity and specificity. Initial treatment of neurocysticercosis should focus on symptom control (e.g., antiepileptic drugs for seizures, neurosurgery for hydrocephalus), not on immediate initiation of anthelminthic therapy. Once symptoms are controlled, anthelminthic therapy should be individualized and take into account the patient's symptoms and the number, viability, and location of cysticerci.13 Treatment, usually with albendazole, can cause a severe inflammatory reaction by killing viable cysts; corticosteroids should be used to suppress this response.14
Persons diagnosed with cysticercosis, as well as their close contacts, should be screened for taeniasis and treated, if necessary. Strategies to prevent cysticercosis include washing hands, raw vegetables, and fruits before eating. Persons traveling to endemic areas should drink only boiled or bottled water and eat only fruits and vegetables that have been cooked or that they have peeled themselves.
Toxoplasmosis
Toxoplasmosis is caused by the parasite Toxoplasma gondii. Humans often acquire Toxoplasma infection by eating undercooked contaminated meat; by eating food contaminated by utensils or cutting boards that have been in contact with raw contaminated meat; by ingesting soil contamined with cat feces on raw fruits or vegetables; or by drinking contaminated water (Figure 4).15 Transmission via blood transfusion or organ transplantation can also occur. Cats are often infected with T. gondii and can shed the parasite in their feces for several weeks when they are newly infected. Cleaning a cat's litter box is a high-risk activity. Congenital transmission usually occurs when a woman is newly infected with T. gondii during or just before pregnancy.
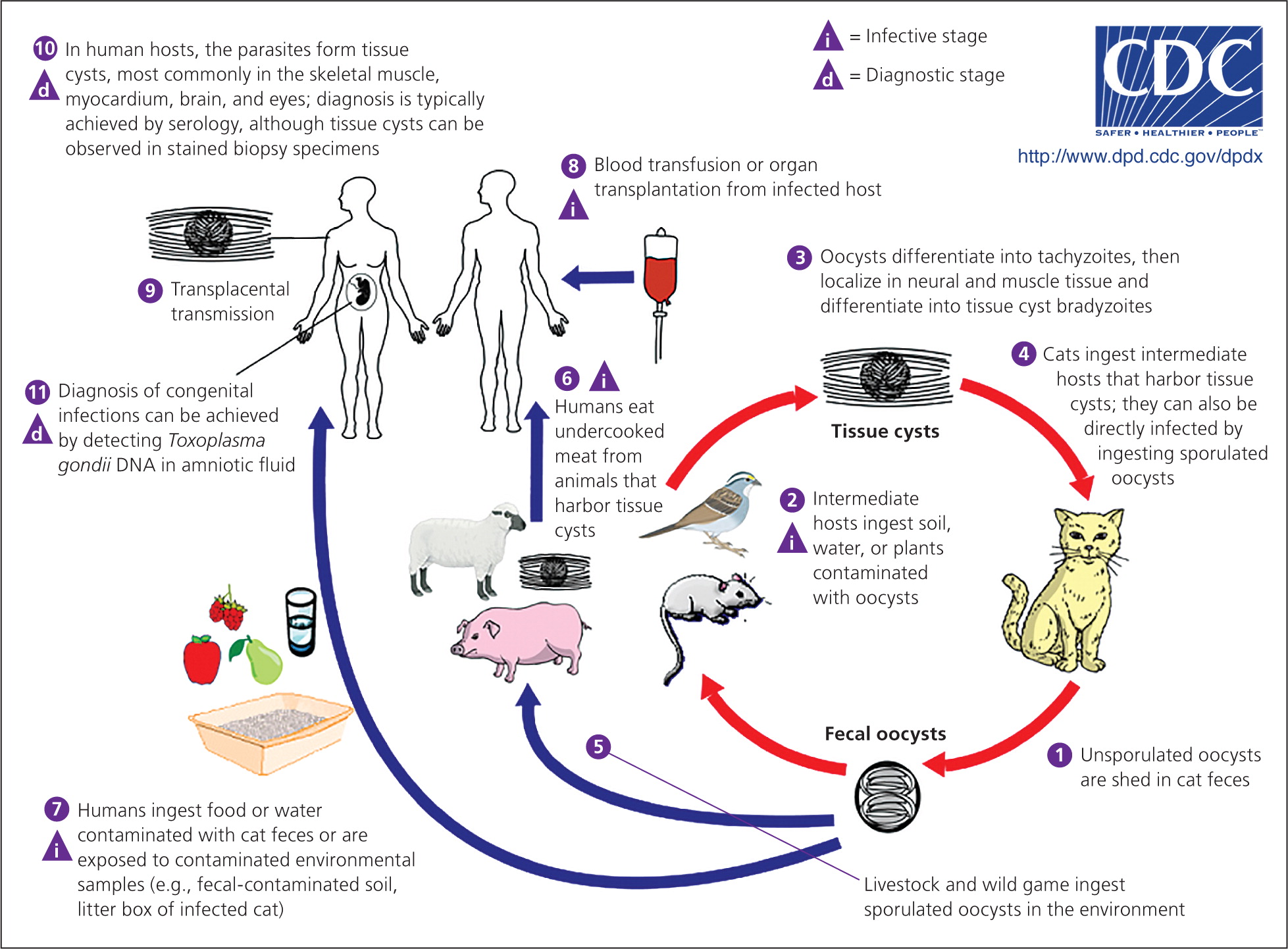
More than 60 million persons in the United States are infected with Toxoplasma, although most immunocompetent persons remain asymptomatic.16 Some experience mild flu-like symptoms, including myalgias or lymphadenopathy, that typically resolve over several weeks. Congenital infections can result in miscarriage or a child born with signs of toxoplasmosis (e.g., jaundice, macrocephaly, microcephaly, seizures). However, infected infants are often asymptomatic at birth.
Immunosuppressed patients who are infected with T. gondii may have severe symptoms of encephalitis, including fever, headache, seizures, confusion, or poor coordination, usually from reactivation of a previous infection. Ocular disease, usually retinochoroiditis, may also develop; patients may present with blurred vision, eye pain, and photophobia. Ocular disease develops in 20% to 80% of persons with congenital infection, but signs are sometimes not present until adulthood.17 In the United States, less than 2% of persons who are infected with T. gondii after birth develop ocular disease.17
Serologic tests that detect Toxoplasma antibodies are available. Immunoglobulin M (IgM) is useful in determining whether infection is recent; IgG reflects chronic infection but may also be present in acute infections. Because false-positive results may occur with IgM testing, it should generally be ordered with an IgG test. The Toxoplasma avidity test can also help determine the timing of infection. Other diagnostic tests (e.g., polymerase chain reaction testing; direct observation of the parasite in blood, tissue, or cerebrospinal fluid) may be useful, especially in immunosuppressed patients in whom the diagnosis may be difficult to confirm. If congenital transmission is a concern, polymerase chain reaction testing can be performed on amniotic fluid as early as 18 weeks' gestation.
Treatment of immunocompetent adults is rarely warranted; if visceral disease is present or symptoms are severe, pyrimethamine (Daraprim), sulfadiazine, and leucovorin may be given for two to four weeks. Immunosuppressed persons with toxoplasmosis require continuous treatment until their immunologic state has improved.18 Women who are infected during the first or early second trimester of pregnancy should be treated with spiramycin.19 When fetal infection is confirmed by amniocentesis, or for infection occurring in the late second or third trimester, pyrimethamine, sulfadiazine, and leucovorin are usually given; infants with congenitally acquired infection should also receive pyrimethamine, sulfadiazine, and leucovorin.18 Pregnant women and immunosuppressed persons should not change cat litter boxes or adopt stray cats. To prevent toxoplasmosis, meat should be cooked thoroughly and items that have been in contact with raw meat should be washed.
Additional Resources
Further information about Chagas disease, toxocariasis, cysticercosis, and toxoplasmosis is available through the Centers for Disease Control and Prevention's website (http://www.cdc.gov/parasites/npi.html). Resources include fact sheets for patients and physicians, continuing medical education courses, and audio podcasts for each disease. Consultation with experts and information about diagnostic testing and investigational drugs are available through the Parasitic Diseases Branch Public Inquiries desk (telephone: 404-718-4745; e-mail: parasites@cdc.gov).
Data Sources: A PubMed search was completed in Clinical Queries using the key terms epidemiology, diagnosis, treatment, and United States for each of the following diseases: Chagas disease, cysticercosis, toxocariasis, and toxoplasmosis. The search included meta-analyses, randomized controlled trials, clinical trials, and reviews. Search dates: October 2012 through February 2014.
