
Am Fam Physician. 2014;90(8):576-578
Author disclosure: No relevant financial affiliations.
Phentermine/topiramate (Qsymia) combines a centrally acting appetite suppressant (phentermine) with an antiepileptic agent (topiramate) in an extended-release capsule. It is labeled for use as an adjunct to exercise and a reduced-calorie diet for chronic weight management in adults with a body mass index (BMI) of 27 kg per m2 or greater and at least one weight-related comorbidity, or with a BMI of at least 30 kg per m2.1 Phentermine/topiramate is a controlled substance (U.S. Food and Drug Administration [FDA] schedule IV).
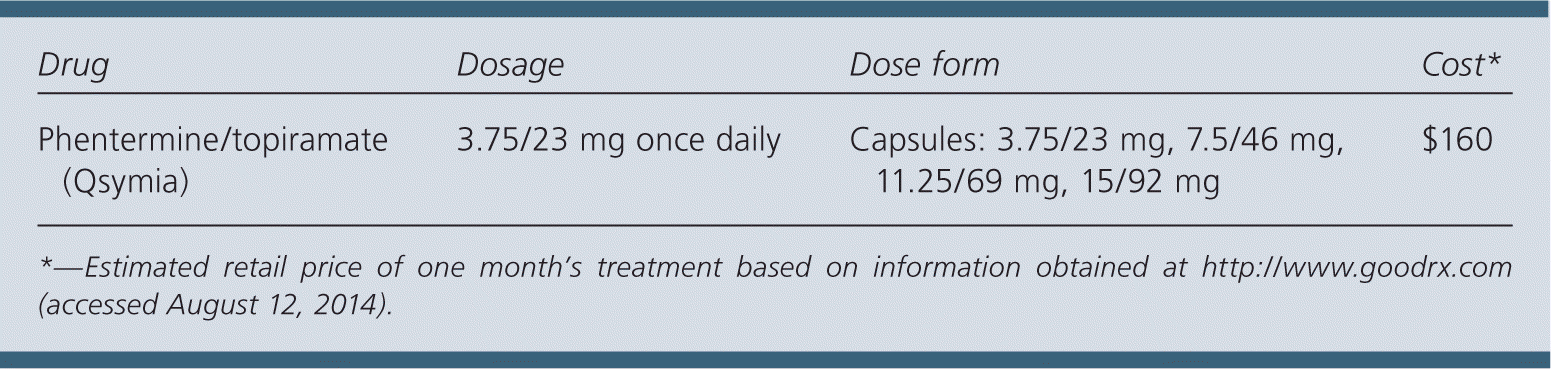
| Drug | Dosage | Dose form | Cost* |
|---|---|---|---|
| Phentermine/topiramate (Qsymia) | 3.75/23 mg once daily | Capsules: 3.75/23 mg, 7.5/46 mg, 11.25/69 mg, 15/92 mg | $160 |
SAFETY
Potential safety issues with phentermine/topiramate include nephrolithiasis, cardiac risk, and teratogenicity. In two 56-week randomized controlled trials with a total of more than 3,500 participants, patients taking phentermine/topiramate did not have more serious adverse events than those taking placebo, with one exception: 1% of patients taking the highest dose (15/92 mg) developed significant nephrolithiasis. Phentermine/topiramate does not increase symptoms of depression, even in patients with preexisting major depression. It does not increase the risk of arrhythmias, valve disease, or myocardial infarction, although studies to date excluded patients with known cardiac problems. Because patients had a dose-dependent increase in heart rate, the FDA is requiring postmarketing evaluation of cardiovascular safety. Phentermine/topiramate is an FDA pregnancy category X drug. In animal and human studies, topiramate has been associated with a risk of cleft lip.
TOLERABILITY
Phentermine/topiramate is well tolerated by most patients. Common side effects are dose dependent and include dry mouth, constipation, paresthesia, insomnia, irritability, and altered taste sensation. These effects typically develop in less than 10% of patients at the lowest dose (3.75/23 mg), 15% at the recommended dose (7.5/46 mg), and between 10% and 20% at the highest dose (15/92 mg). In clinical trials, patients taking phentermine/topiramate were less likely to drop out than patients taking placebo.
EFFECTIVENESS
Combining phentermine/topiramate with a reduced-calorie diet results in greater weight loss than combining reduced calories and increased activity. After one year of treatment, patients taking the maximum dose (15/92 mg) will have lost an average of 11% of their body weight, and those taking the lower dose (7.5/46 mg) will have lost an average of 7% to 8% of their weight. To achieve 5% weight loss (the threshold for clinically meaningful weight loss), three to four patients need to be treated at the lower dose and two to three patients treated at the higher dose for 56 weeks for one additional patient to reach this threshold (number needed to treat [NNT] = 3 to 4, and NNT = 2 to 3, respectively). To achieve 10% weight loss, four to nine patients need to be treated at the lower dose and three patients treated at the higher dose for 56 weeks for one additional patient to reach this threshold (NNT = 4 to 9, and NNT = 3, respectively).
The benefit of this weight loss is not known. Over two years, systolic and diastolic blood pressure will be lowered by 3 to 5 mm Hg, and in patients with type 2 diabetes mellitus, A1C levels will be lowered by 0.2% to 0.4%.2 Patients with type 2 diabetes and those with hypertension may require less medication to control these conditions.2 Patient-oriented outcomes such as the development of osteoarthritis, diabetes, hypertension, and mortality have not been studied. It is unknown if weight loss is sustained after stopping the medication.
PRICE
A 30-day supply of phentermine/topiramate (3.75/23 mg) costs approximately $160. Other FDA-approved medications for the treatment of obesity include lorcaserin (Belviq; $213 for a 30-day supply), orlistat (Xenical; $470), and phentermine ($16).
SIMPLICITY
Patients should take phentermine/topiramate once a day in the morning, with or without food, to lessen the likelihood of insomnia. Patients are instructed to start at the lowest daily dosage (3.75/23 mg) and increase after 14 days to the target dosage (7.5/46 mg per day). If patients do not lose 3% of their body weight after 12 weeks, the dosage should be increased to 11.25/69 mg once daily for another 14 days before increasing to the maximum dosage of 15/92 mg per day. Patients should stop taking phentermine/topiramate after 12 weeks at this dose if they have not lost 5% of their body weight.
Women of childbearing age should have a negative pregnancy test before starting treatment and should continue to have monthly testing during treatment. They should use reliable contraception and discontinue treatment immediately if they conceive.1 Phentermine/topiramate can be dispensed only by certified pharmacies, whose pharmacists are required to give women appropriate counseling regarding pregnancy prevention. Physician training and a list of certified pharmacies are available online.3
Bottom Line
Phentermine/topiramate has been shown to be a good option for sustained weight loss in patients who are unable to lose weight with diet and exercise alone. It should not be given to patients with a history of significant heart disease, and women of childbearing age should use contraception and have regular pregnancy tests when taking phentermine/topiramate.
