A more recent article on tinea infections is available.
Am Fam Physician. 2014;90(10):702-711
Author disclosure: No relevant financial affiliations.
Tinea infections are caused by dermatophytes and are classified by the involved site. The most common infections in prepubertal children are tinea corporis and tinea capitis, whereas adolescents and adults are more likely to develop tinea cruris, tinea pedis, and tinea unguium (onychomycosis). The clinical diagnosis can be unreliable because tinea infections have many mimics, which can manifest identical lesions. For example, tinea corporis can be confused with eczema, tinea capitis can be confused with alopecia areata, and onychomycosis can be confused with dystrophic toe-nails from repeated low-level trauma. Physicians should confirm suspected onychomycosis and tinea capitis with a potassium hydroxide preparation or culture. Tinea corporis, tinea cruris, and tinea pedis generally respond to inexpensive topical agents such as terbinafine cream or butenafine cream, but oral antifungal agents may be indicated for extensive disease, failed topical treatment, immunocompromised patients, or severe moccasin-type tinea pedis. Oral terbinafine is first-line therapy for tinea capitis and onychomycosis because of its tolerability, high cure rate, and low cost. However, kerion should be treated with griseofulvin unless Trichophyton has been documented as the pathogen. Failure to treat kerion promptly can lead to scarring and permanent hair loss.
The term tinea means fungal infection, whereas dermatophyte refers to the fungal organisms that cause tinea. Tinea is usually followed by a Latin term that designates the involved site, such as tinea corporis and tinea pedis (Table 1). Tinea versicolor (now called pityriasis versicolor) is not caused by dermatophytes but rather by yeasts of the genus Malassezia. Tinea unguium is more commonly known as onychomycosis. Dermatophytes are usually limited to involvement of hair, nails, and stratum corneum, which are inhospitable to other infectious agents. Dermatophytes include three genera: Trichophyton, Microsporum, and Epidermophyton.
| Clinical recommendation | Evidence rating | References |
|---|---|---|
| Tinea corporis, tinea cruris, and tinea pedis can often be diagnosed based on appearance, but a potassium hydroxide preparation or culture should be performed when the appearance is atypical. | C | 2 |
| Acceptable treatments for tinea capitis, with shorter treatment courses than griseofulvin, include terbinafine (Lamisil) and fluconazole (Diflucan). | A | 14–16 |
| The diagnosis of onychomycosis should generally be confirmed with a test such as potassium hydroxide preparation, culture, or periodic acid–Schiff stain before initiating treatment. | C | 27 |

| Dermatophytes | |
| Tinea corporis (ringworm), includes tinea gladiatorum and tinea faciei | |
| Tinea capitis (ringworm of the scalp) | |
| Tinea cruris (jock itch) | |
| Tinea pedis (athlete's foot) | |
| Tinea unguium (onychomycosis) | |
| Tinea manuum (commonly presents with “one-hand, two-feet” involvement) | |
| Tinea barbae (beard infection in male adolescents and adults) | |
| Tinea incognito (altered appearance of dermatophyte infection caused by topical steroids) | |
| Candida (yeast) and mold, which may cause onychomycosis or coexist in a dystrophic nail | |
| Pityriasis versicolor (formerly tinea versicolor) caused by Malassezia species | |
| Uncommon fungal skin infections that involve other organs (e.g., blastomycosis, sporotrichosis) | |
The most common infections in prepubertal children are tinea corporis and tinea capitis, whereas adolescents and adults are more likely to develop tinea cruris, tinea pedis, and tinea unguium (onychomycosis). Tinea infections can be difficult to diagnose and treat. In one survey, tinea was the skin condition most likely to be misdiagnosed by primary care physicians.1
Tinea Corporis, Tinea Cruris, and Tinea Pedis
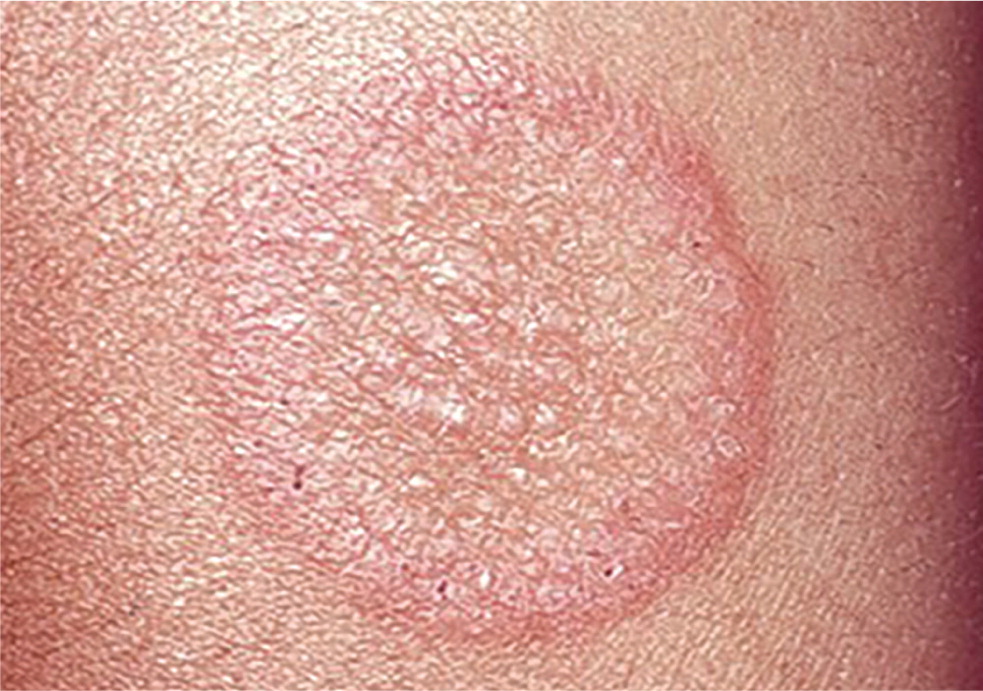
Tinea corporis (ringworm) typically presents as a red, annular, scaly, pruritic patch with central clearing and an active border (Figure 1). Lesions may be single or multiple and the size generally ranges from 1 to 5 cm, but larger lesions and confluence of lesions can also occur. Tinea corporis may be mistaken for many other skin disorders, especially eczema, psoriasis, and seborrheic dermatitis (Table 2).2,3 A potassium hydroxide (KOH) preparation is often helpful when the diagnosis is uncertain based on history and visual inspection. Worsening after empiric treatment with a topical steroid should raise the suspicion of a dermatophyte infection. Conversely, if a nonfungal lesion is treated with an antifungal cream, the lesion will likely not improve or will worsen. Cultures are usually not necessary to diagnose tinea corporis.2 Skin biopsy with periodic acid–Schiff (PAS) stain may rarely be indicated for atypical or persistent lesions.
| Differential diagnosis | Distinguishing features | |
|---|---|---|
| Tinea corporis (annular lesions with well-defined, scaly, often reddish margins; commonly pruritic) | ||
| Annular psoriasis | Gray or silver scale; nail pitting; 70% of affected children have family history of psoriasis2 | |
| Atopic dermatitis | Personal or family history of atopy; less likely to have active border with central clearing; lesions may be lichenified | |
| Erythema multiforme | Target lesions; acute onset; no scale; may have oral lesions | |
| Fixed drug eruption | Dusky; erythematous; usually single, nonscaly lesion; most often triggered by sulfa, acetaminophen, ibuprofen, or antibiotic use | |
| Granuloma annulare | No scale, vesicles, or pustules; nonpruritic; smooth; commonly on dorsum of hands or feet | |
| Lupus erythematosus (subacute cutaneous) | Sun-exposed areas; multiple annular lesions; female-to-male ratio 3:13 | |
| Nummular eczema | More confluent scale; less likely to have central clearing | |
| Pityriasis rosea herald patch | Typically an adolescent with a single lesion on neck, trunk, or proximal extremity; pruritus of herald patch is less common; progression to generalized rash in one to three weeks | |
| Seborrheic dermatitis | Greasy scale on erythematous base with typical distribution involving nasolabial folds, hairline, eyebrows, postauricular folds, chest; annular lesions less common | |
| Tinea cruris (usually occurs in male adolescents and young men; spares scrotum and penis) | ||
| Candidal intertrigo | Involves scrotum; satellite lesions; uniformly red without central clearing | |
| Erythrasma | Red-brown; no active border; coral red fluorescence with a Wood lamp examination | |
| Inverse psoriasis | Red and sharply demarcated; may have other signs of psoriasis such as nail pitting | |
| Seborrheic dermatitis | Greasy scale on erythematous base with typical distribution involving nasolabial folds, hairline, eyebrows, postauricular folds, chest; annular lesions less common | |
| Tinea pedis (rare in prepubertal children; erythema, scale, fissures, maceration; itching between toes extending to sole, borders, and occasionally dorsum of foot; may be accompanied by tinea manuum [“one-hand, two-feet” involvement] or onychomycosis) | ||
| Contact dermatitis | Distribution may match footwear; usually spares interdigital skin | |
| Dyshidrotic eczema | “Tapioca pudding” vesicles on lateral aspects of digits; often involves hands | |
| Foot eczema | May have atopic history; usually spares interdigital skin | |
| Juvenile plantar dermatosis | Shiny taut skin involving great toe, ball of foot, and heel; usually spares interdigital skin | |
| Psoriasis | Involvement of other sites; gray or silver scale; nail pitting; 70% of affected children have family history of psoriasis2 | |
| Tinea capitis (one or more patches of alopecia, scale, erythema, pustules, tenderness, pruritus, with cervical and suboccipital lymphadenopathy; most common in children of African heritage) | ||
| Alopecia areata | Discrete patches of hair loss with no epidermal changes (i.e., no scale); total loss of hair or fine miniature hair growth; exclamation point hairs; no crusting; no inflammation; possible nail pitting | |
| Atopic dermatitis | Personal history or family history of atopy; less often annular; lymphadenopathy uncommon; alopecia less common | |
| Bacterial scalp abscess | Alopecia less likely; hair pluck is painful | |
| Psoriasis | Gray or silver scale; nail pitting; 70% of affected children have family history of psoriasis2; involvement of other sites | |
| Seborrheic dermatitis | Alopecia uncommon; lymphadenopathy uncommon; greasy scale; typical distribution involving nasolabial folds, hairline, eyebrows, postauricular folds, chest | |
| Trichotillomania | No scale; commonly involves eyelashes and eyebrows; hairs of varying lengths | |
| Onychomycosis (discolored [white, yellow, brown], thickened nail with subungual keratinous debris and possible nail detachment; often starting with great toe but can involve any nail) | ||
| Other nail dystrophies, most commonly associated with repeated low-grade trauma, psoriasis, or lichen planus | Appearance can be indistinguishable from onychomycosis; may have other manifestations of alternate diagnosis | |
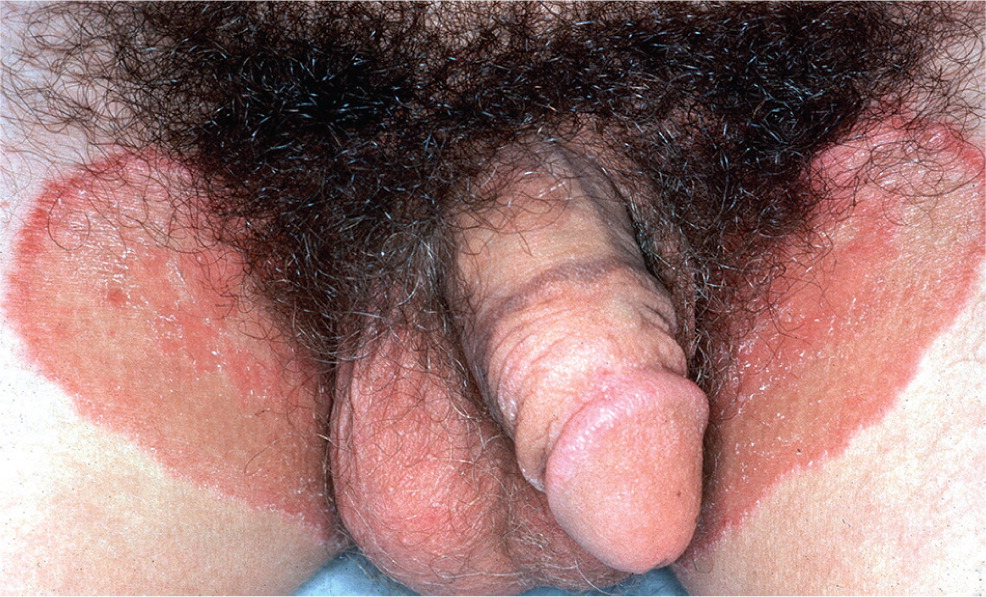
Tinea cruris (jock itch) most commonly affects adolescent and young adult males, and involves the portion of the upper thigh opposite the scrotum (Figure 2). The scrotum itself is usually spared in tinea cruris, but involved in candidiasis. A Wood lamp examination may be helpful to distinguish tinea from erythrasma because the causative organism of erythrasma (Corynebacterium minutissimum) exhibits a coral red fluorescence. However, results of the Wood lamp examination can be falsely negative if the patient has bathed recently.
Tinea pedis (athlete's foot) typically involves the skin between the toes, but can spread to the sole, sides, and dorsum of the involved foot (Figure 3). The acute form presents with erythema and maceration between the toes, sometimes accompanied by painful vesicles. The more common chronic form is characterized by scaling, peeling, and erythema between the toes; however, it can spread to other areas of the foot. Involvement of the plantar and lateral aspects of the foot with erythema and hyperkeratosis is referred to as the “moccasin pattern” of tinea pedis.4
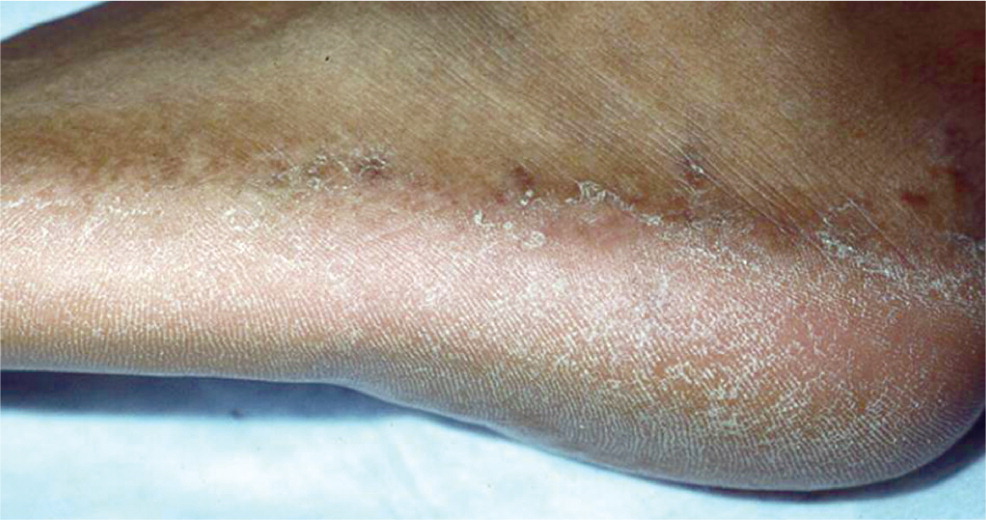
Tinea corporis, tinea cruris, and tinea pedis can often be diagnosed based on appearance, but a KOH preparation or culture should be performed when the appearance is atypical.2
MANAGEMENT
Tinea corporis, tinea cruris, and tinea pedis are generally responsive to topical creams such as terbinafine (Lamisil) and butenafine (Lotrimin Ultra), but oral antifungal agents may be indicated for extensive disease, failed topical treatment, immunocompromised patients, or severe moccasin-type tinea pedis. Patients with chronic or recurrent tinea pedis may benefit from wide shoes, drying between the toes after bathing, and placing lamb's wool between the toes.5 Patients with tinea gladiatorum, a generalized form of tinea corporis seen in wrestlers, should be treated with topical therapy for 72 hours before return to wrestling.6

| Do not use nystatin to treat any tinea infection because dermatophytes are resistant to nystatin. (However, nystatin is often effective for cutaneous Candida infections.) |
| Do not use oral ketoconazole to treat any tinea infection because of the U.S. Food and Drug Administration boxed warnings about hepatic toxicity and the availability of safer agents. |
| Do not use griseofulvin to treat onychomycosis because terbinafine (Lamisil) is usually a better option based on its tolerability, high cure rate, and low cost. |
| Do not use combination products such as betamethasone/clotrimazole because they can aggravate fungal infections. |
| Do not use topical clotrimazole or miconazole to treat tinea because topical butenafine (Lotrimin Ultra) and terbinafine have better effectiveness and similar cost (Table 4). |
| Do not, in general, treat tinea capitis or onychomycosis without first confirming the diagnosis with a potassium hydroxide preparation, culture, or, for onychomycosis, a periodic acid–Schiff stain. However, kerion should be treated aggressively while awaiting test results, and it may be reasonable to treat a child with typical lesions of tinea capitis involving pruritus, scale, alopecia, and posterior auricular lymphadenopathy without confirmatory testing.2,7,8 If there is no lymphadenopathy, a confirmatory test is recommended.2 |
| Do not treat tinea capitis solely with topical agents, but do combine oral therapy with sporicidal shampoos, such as selenium sulfide (Selsun) or ketoconazole. |
| Do not perform potassium hydroxide preparations or cultures on asymptomatic household members of children with tinea capitis, but do consider empiric treatment with a sporicidal shampoo.2 |
Tinea Capitis
In the United States, tinea capitis most commonly affects children of African heritage between three and nine years of age.4 There are three types of tinea capitis: gray patch, black dot, and favus. Black dot, caused by Trichophyton tonsurans, is most common in the United States (Figure 4). Early disease can be limited to itching and scaling, but the more classic presentation involves one or more scaly patches of alopecia with hairs broken at the skin line (black dots) and crusting. Tinea capitis may progress to kerion, which is characterized by boggy tender plaques and pustules. The child with tinea capitis will generally have cervical and suboccipital lymphadenopathy, and the physician may need to broaden the differential diagnosis if lymphadenopathy is absent.7 However, lymphadenopathy can also occur in nonfungal scalp disease, and the absence of lymphadenopathy in an otherwise typical presentation should not delay aggressive treatment for tinea capitis.9
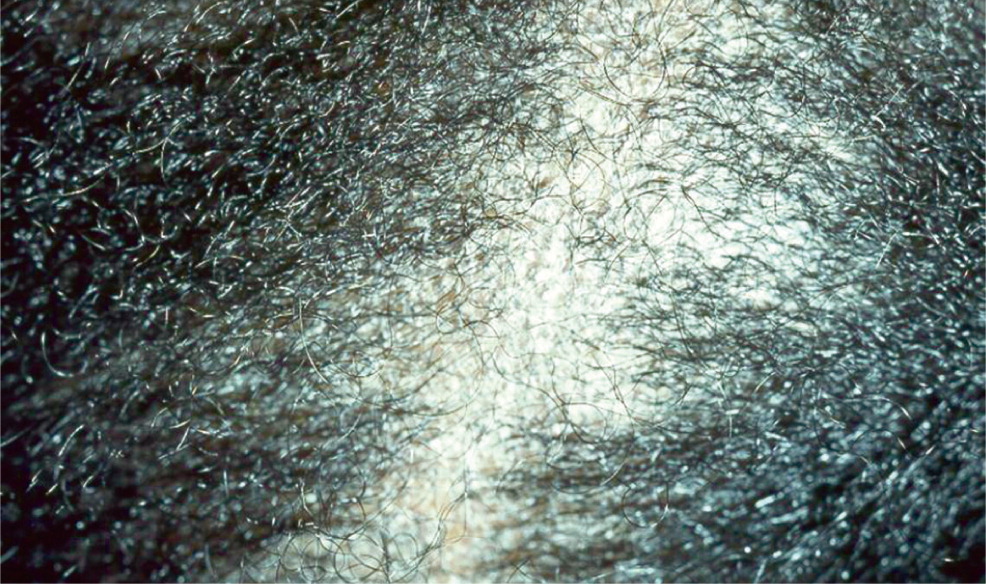
Many physicians treat tinea capitis without a confirmatory culture or KOH preparation if the presentation is typical (i.e., urban setting and child presents with scaling, alopecia, and adenopathy).2,7,8 The most common mimics include seborrheic dermatitis and alopecia areata (Table 2).2,3 In atypical cases, a KOH preparation can be performed by scraping the black dots (broken hairs) and looking for fungal spores. The spores of T. tonsurans will be contained within the hair shaft, but for the less common Microsporum canis, the spores will coat the outside of the hair shaft.
A culture, which is more sensitive than the KOH preparation,10,11 can be performed by moistening a cotton applicator or toothbrush with tap water and rubbing it over the involved scalp. The sample is then applied to Sabouraud liquid medium or Dermatophyte test medium. Children with kerion have a high false-negative culture rate.10 A Wood lamp examination of scalp lesions is often not helpful because the most common cause, T. tonsurans, does not fluoresce. M. canis, which is more common in white children, exhibits a green fluorescence under a Wood lamp. Microsporum infections result from exposure to infected dogs or cats and may produce much more inflammation than Trichophyton infections.4
MANAGEMENT
Tinea capitis must be treated with systemic antifungal agents because topical agents do not penetrate the hair shaft. However, concomitant treatment with 1% or 2.5% selenium sulfide (Selsun) shampoo or 2% ketoconazole shampoo should be used for the first two weeks because it may reduce transmission.12,13 For many years, the first-line treatment for tinea capitis has been griseofulvin because it has a long track record of safety and effectiveness. However, randomized clinical trials have confirmed that newer agents, such as terbinafine and fluconazole (Diflucan), have equal effectiveness and safety and shorter treatment courses14–16 (Table 4).2,12,17–20 Terbinafine may be superior to griseofulvin for Trichophyton species, whereas griseofulvin may be superior to terbinafine for the less common Microsporum species.21,22 Culture results are usually not available for two to six weeks, but 95% of tinea capitis cases in the United States are caused by Trichophyton, making terbinafine a reasonable first choice.23 However, kerion should be treated with griseofulvin unless Trichophyton has been documented as the pathogen.2,17 Failure to treat kerion promptly can lead to scarring and permanent hair loss.2
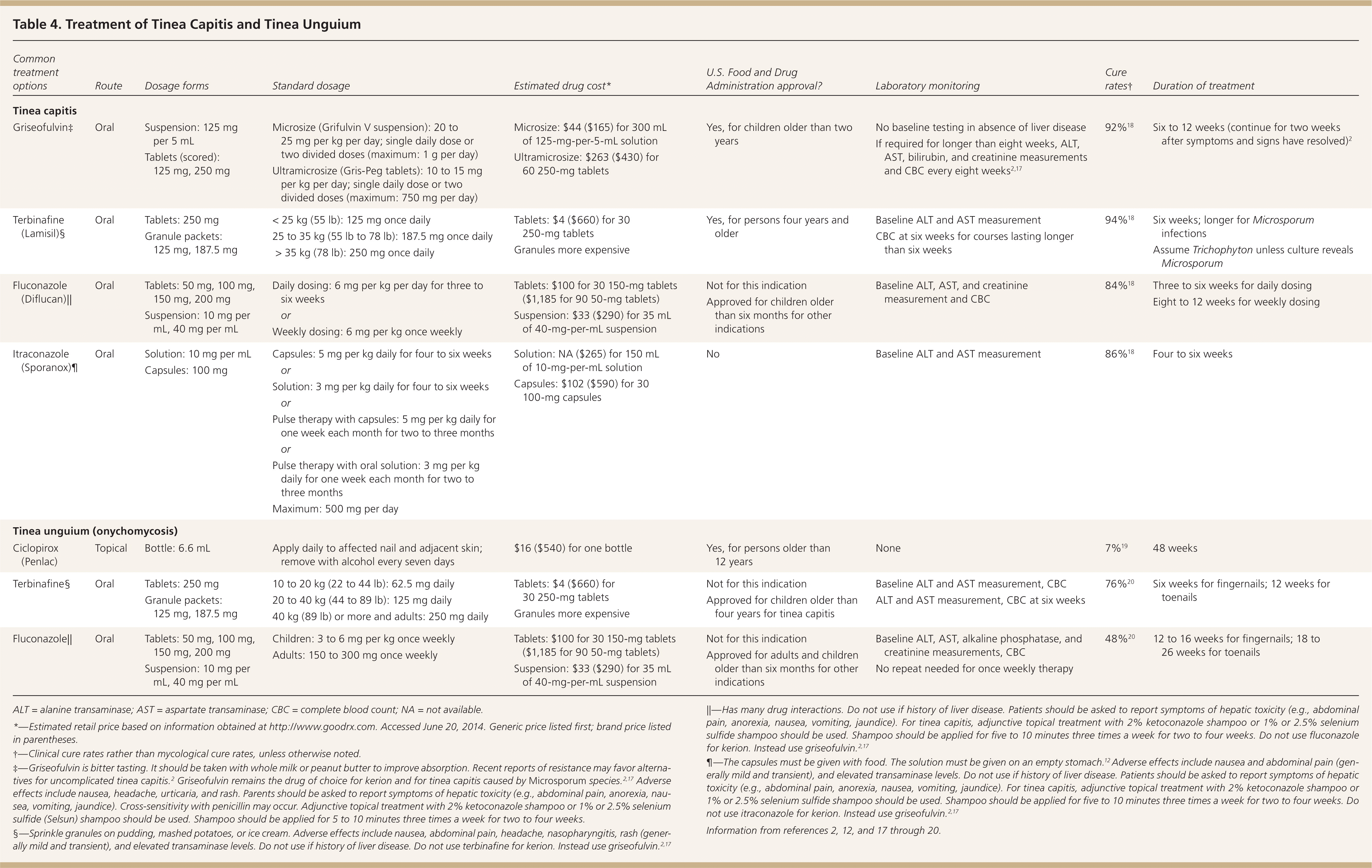
| Common treatment options | Route | Dosage forms | Standard dosage | Estimated drug cost* | U.S. Food and Drug Administration approval? | Laboratory monitoring | Cure rates† | Duration of treatment | |
|---|---|---|---|---|---|---|---|---|---|
| Tinea capitis | |||||||||
|
|
|
|
|
|
|
| ||
|
|
|
|
|
|
|
|
| |
|
|
|
|
|
|
|
|
| |
|
|
|
|
|
|
|
|
| |
| Tinea unguium (onychomycosis) | |||||||||
|
|
|
|
|
|
|
|
| |
|
|
|
|
|
|
|
|
| |
|
|
|
|
|
|
|
|
| |
The child with tinea capitis should return for clinical assessment at the completion of therapy or sooner if indicated, but follow-up cultures are usually unnecessary if there is clinical improvement. Once treatment has started, the child may return to school, but for 14 days should not share combs, brushes, helmets, hats, or pillowcases, or participate in sports that involve head-to-head contact, such as wrestling.2,17 Household members should be clinically evaluated but not necessarily tested for tinea capitis.17 Many experts recommend treating all asymptomatic close contacts with a sporicidal shampoo, such as 2.5% selenium sulfide or 2% ketoconazole, for two to four weeks.2 If children do not improve, parents should be asked about adherence to the treatment regimen. The scalp should also be cultured to identify the organism and immunocompromise should be considered. A second treatment course with the same or a different agent is reasonable if the diagnosis is confirmed.
Tinea Unguium (Onychomycosis)
Onychomycosis is a common consideration in adolescents and adults with dystrophic toenails. In addition to the common distal subungual form, which is characterized by thickened, brittle, discolored nails (Figure 5), onychomycosis may present with an uncommon proximal subungual form, which should raise suspicion of immunocompromise, and a white superficial form, which is more common in children than adults24 (Figure 6).
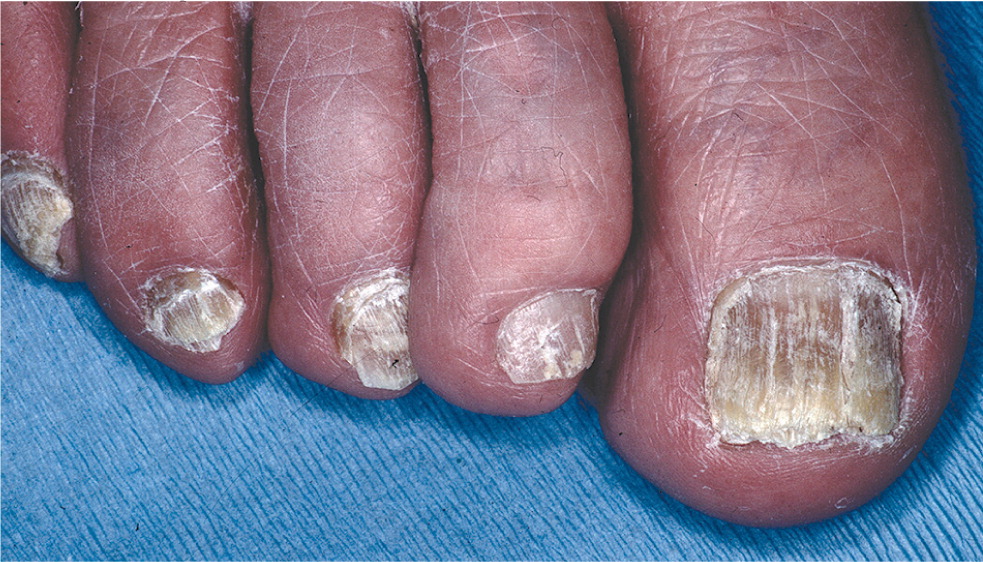
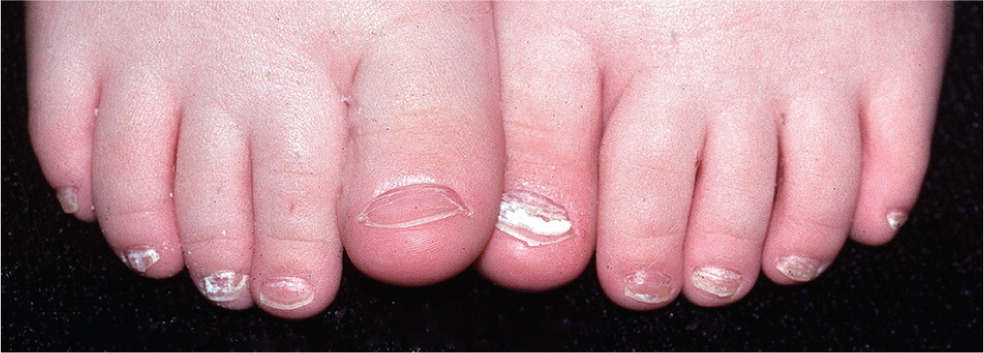
The most common onychomycosis mimics include chronic trauma and psoriasis.25 Adolescents and young adults can develop dystrophic toenails from repeated sudden-stop trauma associated with basketball, soccer, and tennis.26 The great toes are most often involved in onychomycosis and trauma-related dystrophy, but exclusive little toe involvement is likely related to trauma.
The diagnosis of onychomycosis should usually be confirmed with a KOH preparation, culture, or PAS stain because the treatment is long and potentially expensive, and the nonfungal mimics are common.27 In one study, less than 50% of dystrophic toenails resulted in positive fungal cultures.28 However, the involvement of multiple toenails, or accompanying tinea pedis, may justify treatment without confirming the diagnosis.29 The most sensitive diagnostic test, and the most expensive, is the PAS stain,30 which can be performed by placing toenail clippings or curettings in 10% formalin and transporting them to the pathology laboratory. Culture has poor sensitivity, but good specificity.30
MANAGEMENT
Treatment courses for onychomycosis are long (three to six months), failure rates are high (Table 42,12,17–20 ), and recurrences are common (up to 50%).31 In older adults, treatment of onychomycosis is often optional, but most adolescents and young adults request treatment for cosmetic reasons or discomfort from shoes. Topical therapy is usually ineffective except in the treatment of the white superficial form. However, some patients resist systemic treatment, and ciclopirox nail lacquer (Penlac) can be offered together with information about its low cure rate. Oral fluconazole is an option,32 but for most patients oral terbinafine is the treatment of choice because of its superior effectiveness,33 tolerability, and low cost.31,34–38 Because toenails grow slowly, assessment of cure takes nine to 12 months.
KOH Preparation
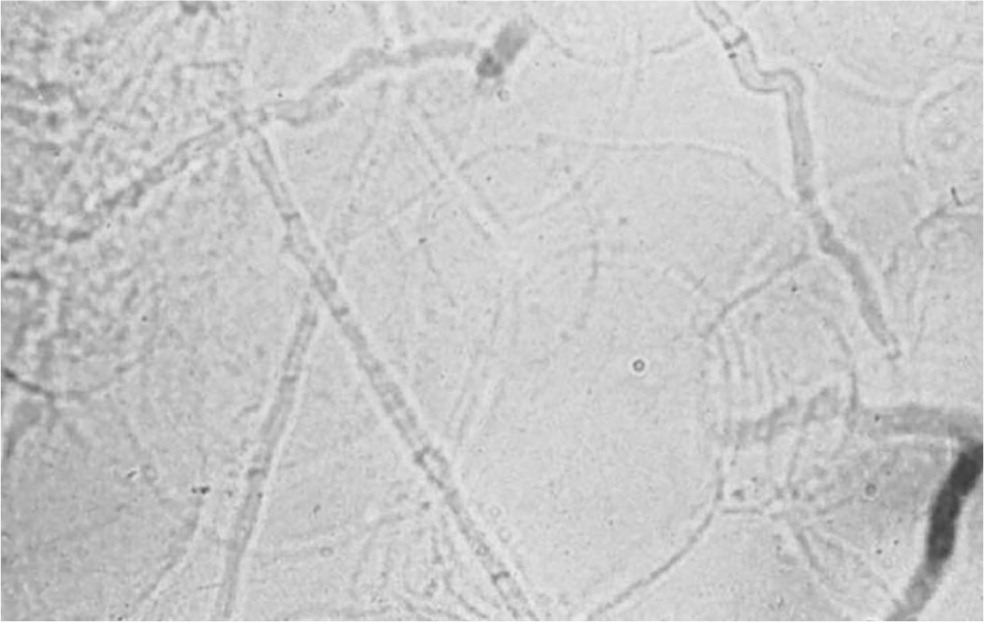
KOH preparations are often needed to confirm the diagnosis of tinea infections (Figure 7). Some tips for performing KOH preparations are available online (eTable A). However, some clinicians may not have immediate access to a microscope or have a Certificate of Provider-Performed Microscopy,39 and transporting skin scrapings to a distant laboratory will not support immediate point-of-care treatment decisions. Even when a microscope is available, the decision to perform an immediate KOH preparation may have to be balanced against other priorities.1,40

| Obtaining the sample |
| The scraping should be taken with a #15 scalpel blade or the edge of a glass slide. The scraped scale should fall onto a microscope slide or into a test tube. False-negative KOH preparations often result from inadequate scrapings. |
| A tinea capitis sample for KOH preparation can be taken by scraping the black dots (hairs broken off at the skin line). |
| For suspected onychomycosis, consider a periodic acid–Schiff stain of nail clippings instead of KOH preparation. |
| Because the scrapings will easily blow off the slide, shield it from drafts or apply KOH preparation to the slide before transport. |
| Preparing the slide |
| Place two drops of 10% or 20% KOH on the scrapings, followed by a coverslip. Alternatively, place a coverslip over the dry scrapings and a drop or two of KOH next to the coverslip and allow it to run under the coverslip. KOH dissolves squamous cells but leaves the fungal elements intact. |
| Heat the slide with a match or alcohol lamp. The match may leave a smoky deposit on the slide. Avoid boiling the KOH, but the slide should be hot enough to be uncomfortable to the dorsum of the hand, usually three to four seconds over the flame. Skin scrapings and hair can be examined under the microscope immediately. Toenail curettings should wait at least 10 minutes to several hours before examination. |
| After heating the slide, tap down the coverslip to compress the sample and separate the hyphae from the squamous cells. |
| Examining the slide under the microscope |
| Adjust the light filter and drop the condenser to achieve a low light level and increased refraction. |
| Scan the slide under low power, and use high power to confirm hyphae in suspicious areas. |
| False-negative results on KOH preparations are common and are usually caused by inadequate material on the slide. False-positive results can occur from misinterpretation of hair shafts or clothing fibers, which are often larger than hyphae, not segmented, and not branching. The borders between squamous cells can also be mistaken for hyphae. |
| The shelf life of a bottle of KOH is at least five years. KOH can damage microscope lenses. Therefore, use an old microscope, and avoid spills and excess KOH on the slide. |
The sensitivity of the KOH preparation varies widely in different settings, ranging from 12% in a study of 27 Flemish general practitioners to 88% in a Nova Scotia tertiary care center 41 (Table 510,11,29,30,41–48 ). These considerations may warrant antifungal treatment in the absence of hyphae under the microscope.2 In a European study of 45,000 patients with suspected onychomycosis, general physicians performed a confirmatory test in only 3% of patients and dermatologists in only 40%.40 However, accurate diagnosis is important, especially for onychomycosis and tinea capitis, because these disorders have many mimics and the treatment is prolonged.
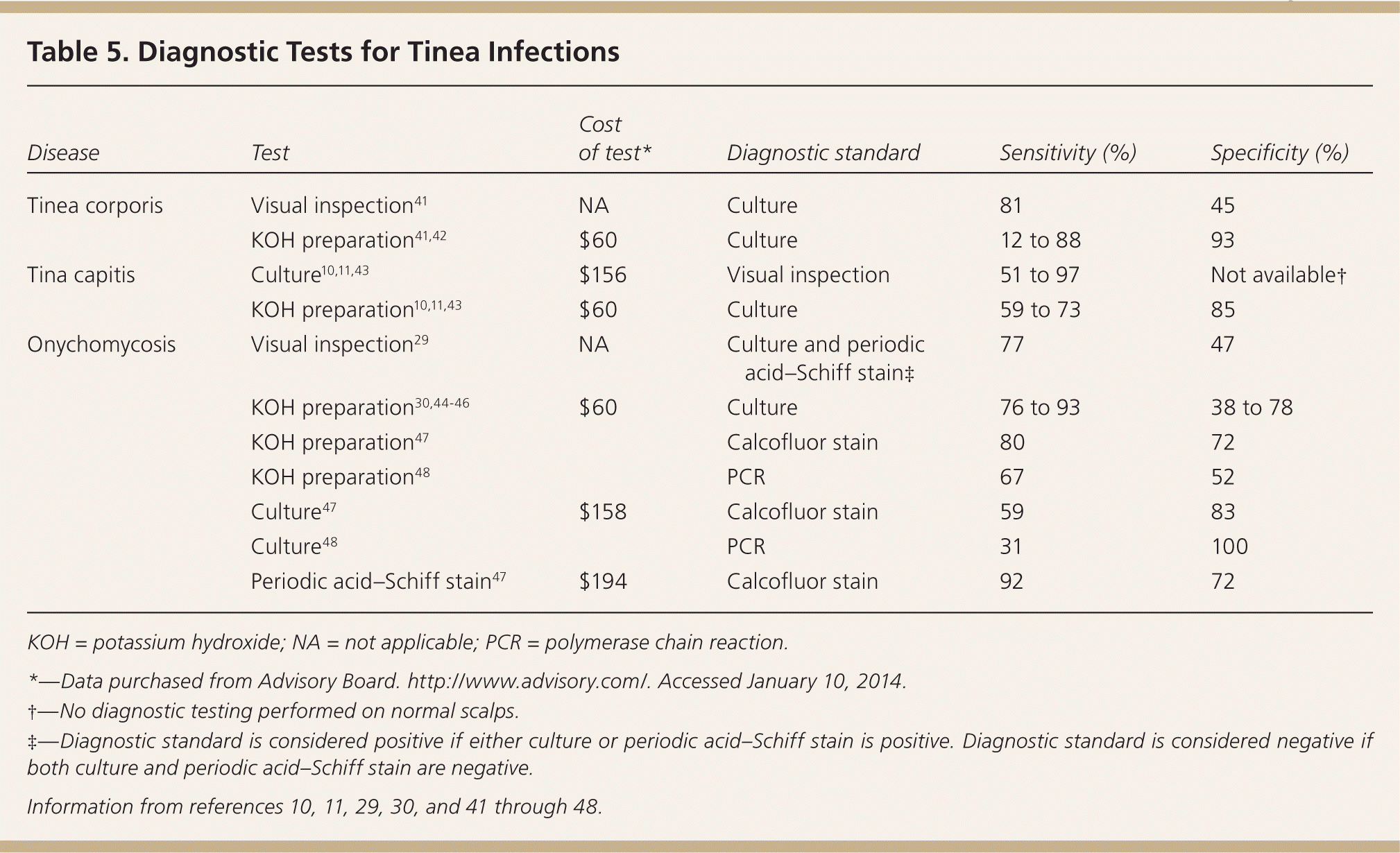
| Disease | Test | Cost of test* | Diagnostic standard | Sensitivity (%) | Specificity (%) |
|---|---|---|---|---|---|
| Tinea corporis | Visual inspection41 | NA | Culture | 81 | 45 |
| KOH preparation41,42 | $60 | Culture | 12 to 88 | 93 | |
| Tina capitis | Culture10,11,43 | $156 | Visual inspection | 51 to 97 | Not available† |
| KOH preparation10,11,43 | $60 | Culture | 59 to 73 | 85 | |
| Onychomycosis | Visual inspection29 | NA | Culture and periodic acid–Schiff stain‡ | 77 | 47 |
| KOH preparation30,44–46 | $60 | Culture | 76 to 93 | 38 to 78 | |
| KOH preparation47 | Calcofluor stain | 80 | 72 | ||
| KOH preparation48 | PCR | 67 | 52 | ||
| Culture47 | $158 | Calcofluor stain | 59 | 83 | |
| Culture48 | PCR | 31 | 100 | ||
| Periodic acid–Schiff stain47 | $194 | Calcofluor stain | 92 | 72 |

| Recommendation | Sponsoring organization |
|---|---|
| Don't prescribe oral antifungal therapy for suspected nail fungus without confirmation of fungal infection. | American Academy of Dermatology |
The first Choosing Wisely recommendation from the American Academy of Dermatology is, “Don't prescribe oral antifungal therapy for suspected nail fungus without confirmation of fungal infection.”27 Clinicians who want to confirm the diagnosis of tinea infections before prescribing therapy have several options: (1) send the skin scrapings in a test tube to an off-site laboratory; (2) if feasible, perform the KOH preparation during the patient visit; or (3) substitute a test that involves less physician time, such as a culture or, in the case of onychomycosis, a PAS stain of nail clippings.
Data Sources: A PubMed search was completed using the MeSH heading “Tinea”[Majr] and including meta-analyses, guidelines, randomized controlled trials, and reviews. Also searched were Essential Evidence Plus, the Cochrane Database of Systematic Reviews, and UpToDate. Finally, we performed multiple targeted searches in PubMed and reference lists of previously retrieved studies to fill in remaining information gaps, such as the performance characteristics of laboratory tests used to diagnose fungal infections. Search dates: October 16, 2013, through July 16, 2014.