
Am Fam Physician. 2015;91(7):online
As published by the U.S. Preventive Services Task Force.
Summary of Recommendations and Evidence
The USPSTF recommends screening for chlamydia in sexually active women aged 24 years or younger and in older women who are at increased risk for infection (Table 1). B recommendation.
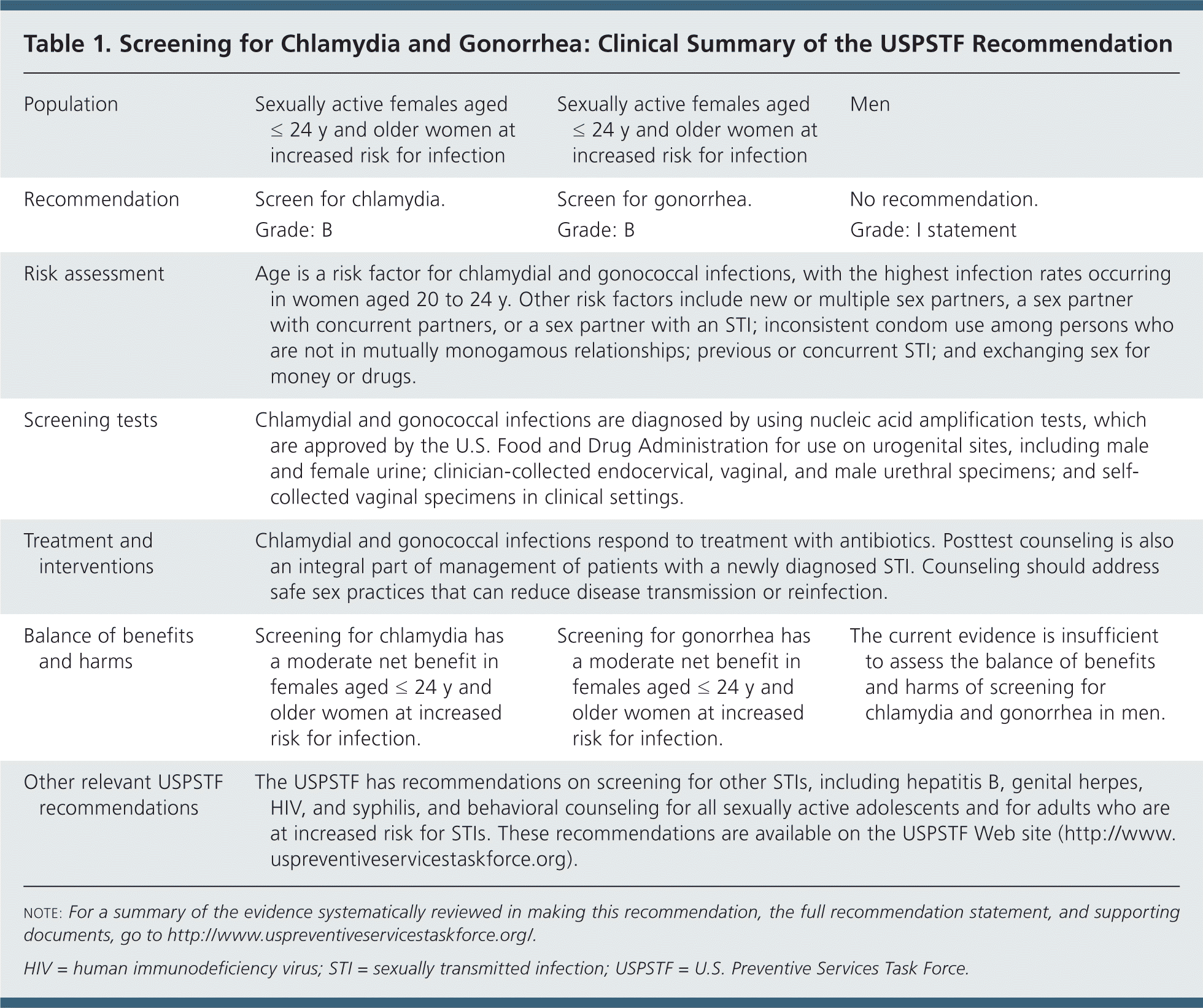
| Population | Sexually active females aged ≤ 24 y and older women at increased risk for infection | Sexually active females aged ≤ 24 y and older women at increased risk for infection | Men | ||
| Recommendation | Screen for chlamydia. | Screen for gonorrhea. | No recommendation. | ||
| Grade: B | Grade: B | Grade: I statement | |||
| Risk assessment | Age is a risk factor for chlamydial and gonococcal infections, with the highest infection rates occurring in women aged 20 to 24 y. Other risk factors include new or multiple sex partners, a sex partner with concurrent partners, or a sex partner with an STI; inconsistent condom use among persons who are not in mutually monogamous relationships; previous or concurrent STI; and exchanging sex for money or drugs. | ||||
| Screening tests | Chlamydial and gonococcal infections are diagnosed by using nucleic acid amplification tests, which are approved by the U.S. Food and Drug Administration for use on urogenital sites, including male and female urine; clinician-collected endocervical, vaginal, and male urethral specimens; and self-collected vaginal specimens in clinical settings. | ||||
| Treatment and interventions | Chlamydial and gonococcal infections respond to treatment with antibiotics. Posttest counseling is also an integral part of management of patients with a newly diagnosed STI. Counseling should address safe sex practices that can reduce disease transmission or reinfection. | ||||
| Balance of benefits and harms | Screening for chlamydia has a moderate net benefit in females aged ≤ 24 y and older women at increased risk for infection. | Screening for gonorrhea has a moderate net benefit in females aged ≤ 24 y and older women at increased risk for infection. | The current evidence is insufficient to assess the balance of benefits and harms of screening for chlamydia and gonorrhea in men. | ||
| Other relevant USPSTF recommendations | The USPSTF has recommendations on screening for other STIs, including hepatitis B, genital herpes, HIV, and syphilis, and behavioral counseling for all sexually active adolescents and for adults who are at increased risk for STIs. These recommendations are available on the USPSTF Web site (http://www.uspreventiveservicestaskforce.org). | ||||
The USPSTF recommends screening for gonorrhea in sexually active women aged 24 years or younger and in older women who are at increased risk for infection. B recommendation.
See the Clinical Considerations section for a description of populations at increased risk for infection.
The USPSTF concludes that the current evidence is insufficient to assess the balance of benefits and harms of screening for chlamydia and gonorrhea in men. I statement.
See the Clinical Considerations section for suggestions for practice regarding the I statement.
Rationale
IMPORTANCE
Chlamydia and gonorrhea are the most commonly reported sexually transmitted infections (STIs) in the United States. In 2012, more than 1.4 million cases of chlamydia and more than 330,000 cases of gonorrhea were reported to the Centers for Disease Control and Prevention (CDC).1 Chlamydial infections are 10 times more prevalent than gonococcal infections (4.7% vs. 0.4%) in women aged 18 to 26 years.2
Although most identified cases are reported, the incidence of chlamydia and gonorrhea is difficult to estimate because most infections are asymptomatic and are therefore never diagnosed. The CDC estimates that more than 800,000 persons are infected with gonorrhea in the United States each year, and fewer than half of these infections are diagnosed and reported.3
Chlamydial and gonococcal infections are often asymptomatic in women; however, asymptomatic infection may lead to pelvic inflammatory disease (PID) and its associated complications, such as ectopic pregnancy, infertility, and chronic pelvic pain. Newborns of women with untreated infection may develop neonatal chlamydial pneumonia or gonococcal or chlamydial ophthalmia. Infection may lead to symptomatic urethritis and epididymitis in men, although gonorrhea is more likely than chlamydia to be symptomatic in men compared with women. Both types of infection may facilitate HIV transmission.1,4,5
DETECTION
The USPSTF found convincing evidence that screening tests can accurately detect chlamydia. The USPSTF also found convincing evidence that screening tests can accurately detect gonorrhea.
BENEFITS OF EARLY DETECTION AND INTERVENTION OR TREATMENT
The USPSTF found adequate direct evidence that screening reduces complications of chlamydial infection in women who are at increased risk, with a moderate magnitude of benefit.
The USPSTF found adequate evidence that screening for gonorrhea results in a moderate magnitude of benefit based on the large proportion of cases that are asymptomatic, the effectiveness of antibiotic treatment to reduce infections, and the high morbidity associated with untreated infections.
The USPSTF found inadequate evidence that screening for chlamydia and gonorrhea reduces complications of infection and transmission or acquisition of either disease or HIV in men. The magnitude of benefit is unknown.
HARMS OF EARLY DETECTION AND INTERVENTION OR TREATMENT
The USPSTF found adequate evidence that the harms of screening for chlamydia and gonorrhea are small to none.
USPSTF ASSESSMENT
The USPSTF concludes with moderate certainty that screening for chlamydia is associated with moderate net benefit in all sexually active women aged 24 years or younger and in older women who are at increased risk for infection.
The USPSTF concludes with moderate certainty that screening for gonorrhea is associated with moderate net benefit in all sexually active women aged 24 years or younger and in older women who are at increased risk for infection.
The USPSTF concludes that the current evidence is insufficient to assess the balance of benefits and harms of screening for chlamydia and gonorrhea in men.
Clinical Considerations
PATIENT POPULATION UNDER CONSIDERATION
This recommendation applies to all sexually active adolescents and adults, including pregnant women.
ASSESSMENT OF RISK
Age is a strong predictor of risk for chlamydial and gonococcal infections, with the highest infection rates occurring in women aged 20 to 24 years, followed by females aged 15 to 19 years. Chlamydial infections are 10 times more prevalent than gonococcal infections in young adult women.2 Among men, infection rates are highest in those aged 20 to 24 years.1
Other risk factors for infection include having a new sex partner, more than 1 sex partner, a sex partner with concurrent partners, or a sex partner who has an STI; inconsistent condom use among persons who are not in mutually monogamous relationships; previous or coexisting STI; and exchanging sex for money or drugs. Prevalence is also higher among incarcerated populations, military recruits, and patients receiving care at public STI clinics. There are also racial and ethnic differences in STI prevalence. In 2012, black and Hispanic persons had higher rates of infection than white persons.1 Clinicians should consider the communities they serve and may want to consult local public health authorities for guidance on identifying groups that are at increased risk. Gonococcal infection, in particular, is concentrated in specific geographic locations and communities.
SCREENING TESTS
Chlamydia trachomatis and Neisseria gonorrhoeae infections should be diagnosed by using nucleic acid amplification tests (NAATs) because their sensitivity and specificity are high and they are approved by the U.S. Food and Drug Administration for use on urogenital sites, including male and female urine, as well as clinician-collected endocervical, vaginal, and male urethral specimens.6 Most NAATs that are approved for use on vaginal swabs are also approved for use on self-collected vaginal specimens in clinical settings. Rectal and pharyngeal swabs can be collected from persons who engage in receptive anal intercourse and oral sex, although these collection sites have not been approved by the U.S. Food and Drug Administration.7 Urine testing with NAATs is at least as sensitive as testing with endocervical specimens, clinician- or self-collected vaginal specimens, or urethral specimens that are self-collected in clinical settings. The same specimen can be used to test for chlamydia and gonorrhea.7
SCREENING INTERVALS
In the absence of studies on screening intervals, a reasonable approach would be to screen patients whose sexual history reveals new or persistent risk factors since the last negative test result.
TREATMENT AND INTERVENTIONS
Chlamydial and gonococcal infections respond to treatment with antibiotics. Centers for Disease Control and Prevention guidelines for treatment of sexually transmitted diseases (STDs) and expedited partner therapy are available at http://www.cdc.gov/std/treatment/2010/default.htm and http://www.cdc.gov/std/ept/default.htm, respectively.
Posttest counseling is an integral part of management of patients with a newly diagnosed STI. The USPSTF recommends offering or referral to high-intensity behavioral counseling for patients with current or recent STIs (http://www.uspreventiveservicestaskforce.org/uspstf/uspsstds.htm). Posttest counseling can also serve as an educational opportunity for patients who present with STI concerns but test negative for infection. It should address safe sex practices that can reduce disease transmission or reinfection; motivational interviewing strategies may also promote risk-reducing behaviors.
To maximize adherence, the CDC recommends that drug treatment be dispensed on site. The CDC recommends that all sex partners of infected patients from the preceding 60 days be evaluated, tested, and treated for infection. It also recommends that infected patients be instructed to abstain from sexual intercourse until after they and their sex partners have completed treatment and no longer have symptoms. For a sex partner who cannot be linked to care, the CDC suggests that clinicians consider expedited partner therapy, which allows for the delivery of a drug or drug prescription to the partner by the patient, a disease investigation specialist, or a pharmacy. Because of a high likelihood of reinfection, the CDC also recommends retesting all patients diagnosed with chlamydial or gonococcal infection 3 months after treatment, regardless of whether they believe their partners have been treated.
In pregnant women, a test of cure to document eradication of chlamydial infection 3 weeks after treatment is recommended. Pregnant women diagnosed with a chlamydial or gonococcal infection in the first trimester should be retested 3 months after treatment. Gonococcal neonatal ophthalmia, which can be transmitted from an untreated woman to her newborn, may be prevented with routine topical prophylaxis at delivery. However, prevention of chlamydial neonatal pneumonia and ophthalmia requires prenatal detection and treatment.
SUGGESTIONS FOR PRACTICE REGARDING THE I STATEMENT
Potential Preventable Burden. Chlamydial and gonococcal infections are often asymptomatic in men but may result in urethritis, epididymitis, and proctitis. Uncommon complications include reactive arthritis (chlamydia) and disseminated gonococcal infection. Infections at extragenital sites (such as the pharynx and rectum) are typically asymptomatic. Chlamydial and gonococcal infections may facilitate HIV transmission in men and women.1,4,5 Median prevalence rates among men who have sex with men who were tested in STD Surveillance Network clinics in 2012 were 16% for gonorrhea and 12% for chlamydia.1
Potential Harms. Potential harms of screening for chlamydia and gonorrhea include false-positive or false-negative results as well as labeling and anxiety associated with positive results.
Costs. According to the CDC, STIs in the United States are associated with an annual cost of almost $16 billion.8 Among nonviral STIs, chlamydia is the most costly, with total associated costs of $516.7 million (range, $258.3 to $775.0 million). Gonococcal infections are associated with total costs of $162.1 million (range, $81.1 to $243.2 million).9
In 2008, estimated direct lifetime costs (in 2010 U.S. dollars) per case of chlamydial infection were $30 (range, $15 to $45) in men and $364 (range, $182 to $546) in women. Similarly, gonococcal infections were associated with direct costs of $79 (range, $40 to $119) in men and $354 (range, $182 to $546) in women.9
Current Practice. A review of health care claims of 4,296 male and female patients presenting for general medical or gynecologic examinations from 2000 to 2003 found that a large proportion of those with high-risk sexual behaviors did not receive STI or HIV testing during their visit. According to a review of diagnostic billing codes for patients with high-risk sexual behaviors, men were significantly less likely than women to be tested for chlamydia (20.7% vs. 56.9%) and gonorrhea (20.7% vs. 50.9%), although they were more likely to be tested for HIV (79.3% vs. 38.8%) and syphilis (39.1% vs. 27.6%).10
OTHER APPROACHES TO PREVENTION
The USPSTF has issued recommendations on screening for other STIs, including hepatitis B, genital herpes, HIV, and syphilis. The USPSTF has also issued recommendations on behavioral counseling for all sexually active adolescents and for adults who are at increased risk for STIs. These recommendations are available at http://www.uspreventiveservicestaskforce.org.
USEFUL RESOURCES
The CDC provides more information about STDs, including chlamydia and gonorrhea, at http://www.cdc.gov/std/default.htm. Its recommendations for STD prevention include clinical prevention guidance (available at http://www.cdc.gov/std/treatment/2010/clinical.htm) and patient prevention information (available at http://www.cdc.gov/std/prevention/default.htm). The CDC has also issued guidance for clinicians on how to take a sexual history (available at http://www.cdc.gov/std/treatment/SexualHistory.pdf).
The Community Preventive Services Task Force has issued several recommendations on the prevention of HIV/AIDS, other STIs, and teen pregnancy. The Community Guide discusses interventions that have been efficacious in school settings and for men who have sex with men (available at http://www.thecommunityguide.org/hiv/index.html).
Canadian guidelines on STIs are available at http://www.phac-aspc.gc.ca/std-mts/sti-its/cgsti-ldcits/index-eng.php.
